Abstract
Purpose
To identify the risk factors for, and clinical features and treatment outcomes of aggressive posterior retinopathy of prematurity (APROP) in Korean infants.
Methods
Among 770 premature infants who underwent screening, 105 infants (198 eyes, 13.63%) received treatment for ROP. A total of 24 infants (48 eyes, 3.12%) developed APROP while 81 infants (150 eyes, 10.52%) developed non-APROP treatment-requiring type. The medical records of ROP-treated infants were reviewed retrospectively. The associated systemic and maternal risk factors were analyzed and anatomical outcomes were compared according to the severity of ROP and treatment modalities.
Results
The mean gestational age and birth weight at birth in the APROP group were significantly lower than those in the non-APROP group (P=0.019, P<0.001, respectively). Infants who were born small for their GA developed APROP more frequently than non-APROP patients (P<0.001). Chorioamnionitis-positive infants also showed higher incidence rate of APROP (APROP vs non-APROP; P<0.001 and zone I APROP vs posterior zone II APROP; P=0.036, respectively). Infants with APROP required heavier laser treatment with a higher retreatment rate compared to infants with non-APROP. Favorable anatomical outcomes were achieved in 95.3% from treatment-requiring non-APROP group, 85.7% from zone I APROP and 84.6% from posterior zone II APROP group.
Conclusion
Intrauterine growth restriction and chorioamnionitis were associated with development of APROP. These findings suggest that perinatal maternal environment inhibiting normal retinal vascular growth in utero may contribute to increasing the risk of APROP in premature infants.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a proliferative vitreoretinopathy, a leading cause of lifelong visual impairment in premature infants.1 Both CRYO-ROP2 and Early Treatment ROP3 studies demonstrated that low birth weight (BW) and small gestational age (GA) are the most important clinical predictive factors for ROP development. The advancement in neonatal care has led to prolonged survival of extremely premature infants and increased prevalence of zone I ROP.4 Aggressive posterior ROP (APROP), a severe form of ROP, with an incidence rate of 2.5%, is defined as stated in International Classification of ROP (ICROP) study that includes, posterior location (zone I or posterior zone II), increased dilation and tortuosity of the posterior pole vessels in all quadrants out of proportion to the peripheral retinopathy with flat extraretinal fibrovascular proliferation (EFP), and a rapidly progressive course.5 Management of APROP may be complicated due to poor visualization of the fundus by persistent tunica vasculosa lentis or flat growth of neovascularization along the retina. However, despite appropriate laser ablation, persistent or recurrent vascular activity and retinal detachment may take place, requiring second-stage laser or additional anti-vascular endothelial growth factor (VEGF) treatment.6 The treatment prognosis is reported to be poorer than the typical threshold ROP.7 In this study, we aimed to determine the clinical and demographic risk factors of APROP, looking forward to providing information on factors that predispose individuals to APROP, beyond those that have already been described such as BW and GA. Furthermore, we assessed the response to treatment, number of retreatments required, and final anatomical outcomes.
Materials and methods
This study was approved by the Institutional Review Board of Seoul St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea (KC15RISE0815).
A retrospective study was performed at Seoul St Mary’s Hospital (Seoul, Republic of Korea) from January 2009–December 2014. From the start of our study, the criteria for screening were as follows: BW ≤2000 g or GA ≤34 weeks. A total of 770 premature infants (1440 eyes) were screened. The first examination was scheduled when the infant was 31–33 weeks GA or post-natal 4–6 weeks old, whichever was first. Subsequent examinations and treatments were planned following the Early Treatment ROP (ETROP) recommendations. The ocular findings were classified according to the ICROP criteria.
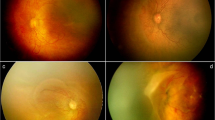
Among the premature infants who underwent screening, 105 infants (198 eyes, 13.63%) required treatment for ROP. The criteria for treatment included the following parameters as defined by the ETROP study: zone I any stage of ROP with plus disease, zone I stage 3 without plus disease, and zone II stage 2 or 3 with plus disease. Patients were then divided into three groups as follows: the APROP groups with ill-defined flat neovascularization, regardless of stage; zone I APROP (Figure 1a), posterior zone II APROP (Figure 1b), and treatment-requiring non-APROP group that refers to plus disease and the presence of EFP extending from ridge (more than five contiguous or eight cumulative clock hours) (Figure 1c). All cases of ROP requiring treatment underwent laser photocoagulation on the entire avascular retina as a first-line treatment. Meanwhile, 14 infants with rapid disease progression, such as zone I or posterior zone II APROP received additional intravitreal anti-VEGF (0.25 mg/0.01 ml of bevacizumab) injection simultaneously with laser photocoagulation or postoperatively, according to disease severity.
Zone I APROP (a) and posterior zone II APROP (b) showing peripheral ill-defined retinopathy and flat neovascularization at the junction of the vascular and avascular retina and circumferential intraretinal shunting. Non-APROP treatment-requiring ROP (c) shows plus disease and stage 3 extraretinal fibrovascular proliferation (EFP) extending from ridge in zone I and zone II, consistent with the criteria threshold defined in the CRYO-ROP study.
Infants were followed up carefully until disease stabilization with regression of plus sign and EFP was achieved. Thereafter, regular follow-up was carried out at postoperative 3 months, 6 months, 1 year, and 3 years. Visual acuity, ocular alignment, and fundus were checked at every visit while the cycloplegic refractive measurement was determined starting at postoperative 1 year.
The associated risk factors for the development of ROP were analyzed. Possible systemic risk factors associated with ROP were collected, including the Apgar score at 1 and 5 min, intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), patent ductus arteriosus (PDA), sepsis, antibiotic use, duration of oxygen supplementation and assisted ventilation or continuous positive airway pressure (CPAP), thrombocytopenia (defined as a platelet count <100 × 109/l), mean platelet count, and the number of platelet transfusions. Platelet data available within 1 week prior to ROP treatment were analyzed in this study. Possible maternal risk factors included maternal age, premature rupture of membrane (PROM), preeclampsia, and chorioamnionitis. Chorioamnionitis was defined by microbiologic and histologic assessment: (a) positive for Ureaplasma urealyticum or Mycoplasma hominis in maternal vaginal swab, placental tissue, or infant gastric juice; (b) presence of acute histologic changes on examination of the amniotic membrane and chorion of the placenta and umbilical vessel wall, characterized by leukocyte infiltration.8, 9, 10
Treatment outcomes and retreatment rates were also documented. Anatomical outcome was evaluated by fundus photography, ultrasonography, and B-scan. Posterior segments without macular dragging, retinal fold, or retinal detachment, were considered as favorable anatomical outcomes.
Statistical analysis was performed using the Student’s t-test and Pearson’s χ2-test to compare the demographic characteristics and the frequency of risk factors between groups, with the SPSS Statistics 19.0 software (IBM Corporation, Armonk, NY, USA). The patients of each group were randomly selected to be matched for their GA, the possible confounder in the development of ROP. For all analyses, a P-value <0.05 was considered significant.
Results
During 6-years’ period, a total of 770 premature infants (1440 eyes) were screened for ROP. Overall, 105 infants (198 eyes, 13.63%) required treatment for ROP, among which, 81 (150 eyes, 10.52%) developed treatment-requiring non-APROP, while 24 infants (48 eyes, 3.12%) developed APROP. The prevalence of treatment-requiring ROP according to BW is shown in Table 1.
The demographics of the ROP-treated infants are described in Table 2. The mean BW was 0.92±0.44 kg in the APROP group and 1.15±0.30 kg in the non-APROP group (P<0.001). Infants with APROP also had a lower GA at birth (26.99±2.23 weeks) compared to infants who developed non-APROP (28.77±2.93 weeks), the difference of which was statistically significant (P=0.019). Treatment of APROP eyes occurred at a mean post-menstrual age (PMA) of 34.12±3.18 weeks and received a mean of 1558.94±415.23 total laser spots. This was significantly greater than treatment-requiring non-APROP cases, which received a mean total of 798.38±393.61 laser spots (P<0.001) at a greater PMA of 36.30±5.70 weeks (P=0.001). Considering the APROP subgroups, zone I APROP group had a lower BW (P=0.002), lower GA at birth (P=0.029), and earlier PMA at first treatment (P=0.145) with more total laser spots (P<0.001) for treatment, compared with posterior zone II APROP.
The risk factors in infants with APROP (for zone I and posterior zone II) and non-APROP types were evaluated separately (Table 3). As the BW of infants from the APROP group was smaller than non-APROP infants, the number of infants who fell in the category of SGA was greater in the APROP group (P<0.001). The mean Apgar score at 1 and 5 min; the presence of IVH, NEC, BPD, PDA, and sepsis; antibiotic use over 14 days; number of platelet transfusions; and maternal risk factors such as maternal age, PROM, and preeclampsia revealed no statistical significance between overall APROP and non-APROP infants (P>0.05). Likewise, the application of oxygen, with or without mechanical ventilation or CPAP, as well as the total duration of oxygen therapy did not increase the risk of APROP development. However, the incidence of thrombocytopenia was significantly higher in the APROP group with significantly low mean platelet count compared to the non-APROP group (P=0.025 and P=0.006, respectively). APROP subgroup analysis demonstrated higher occurrence of thrombocytopenia in zone I APROP group compared to posterior zone II APROP group (P=0.011). The incidence of chorioamnionitis was significantly higher in the APROP group compared to the non-APROP group (P<0.001). For the APROP subgroup, the incidence rate of chorioamnionitis was higher in zone I APROP infants compared to posterior zone II APROP infants (P=0.036).
To eliminate the effect of prematurity as a confounder, the three groups were matched by GA. As a result, platelet count lost statistical significance (P=0.564). However, the proportion of SGA infants was significantly higher (P<0.001) and chorioamnionitis was more frequent in APROP infants compared to non-APROP infants (P=0.021).
Table 4 shows the anatomical outcomes after treatment according to the patient group and the treatment received. The retreatment rate was higher in the APROP group (16.7%) than in the non-APROP (8%) group. Among infants with APROP, 18 eyes (85.7%) from the zone I APROP group and 22 eyes (84.6%) from the posterior zone II APROP group achieved favorable anatomical outcome, which was lower than that of the treatment-requiring non-APROP group, in which 143 eyes (95.3%) had a favorable outcome. Sequelae such as macular dragging, retinal folding, and retinal detachment were more frequently observed in infants with APROP.
Discussion
The aim of this single-center study was to analyze the risk factors for, and clinical features and treatment outcomes of APROP, compared with treatment-requiring non-APROP-type disease. The major finding of our study was that, in addition to prematurity and low BW, SGA and chorioamnionitis appeared to be the risk factors specific to the development of APROP. Thus, our findings suggest the potential role of perinatal maternal environment responsible for developing severe type ROP.
Infants who develop APROP, which comprise ~1/4 of all infants with treatment-requiring ROP, have significantly lower GA and BW at birth compared with infants with non-APROP-type ROP. In addition, we found that the GA and BW of infants with zone I APROP were even lower than those with posterior zone II APROP. These results are consistent with previous studies, which have included patients with severe ROP as well as APROP.6 Importantly, these findings suggest that lower BW and GA at birth are associated with an earlier onset of ROP which in turn is associated with a higher incidence of APROP.
The overall BW of infants in our study was higher than that reported in previous studies. This may have been due to the screening criteria, applied to our institution, which consisted of infants with a BW ≤2000 g or GA ≤34 weeks from the start of our study, and might have eventually raised the mean BW of study participants. We have experienced treatment-requiring ROP in nine infants over 1500 g BW with a prevalence of 3.82% (Table 1). It should be noted that even two Korean infants over 1500 g BW developed posterior zone II APROP.11
In our study, the proportion of SGA babies was significantly higher in the APROP group compared to the non-APROP group. SGA, defined as BW <10th percentile for GA, increases the perinatal morbidity and mortality rates in premature infants. In addition, a number of studies revealed that SGA is an independent risk factor for ROP development.12, 13 This finding is also supported by experimental animal models, in which growth-restricted rats were found to have more abnormal retinal neovascularization in two well-established models of ROP.14 As SGA infants often have poorer general conditions than their AGA peers, they are more likely to require oxygen supplementation, a well-known risk factor for ROP.15 Other possible mechanisms associated with intrauterine growth restriction include chronic uterine hypoxia, antioxidant deficiency, free oxygen radicals in utero, and abnormal growth factor levels.16
Our study demonstrated that chorioamnionitis is one of the factors that increases the risk of developing APROP. Moreover, within the APROP group in this study, zone I APROP, which is the more severe type, seemed to have stronger connection with chorioamnionitis. The results were still valid after adjusting for GA. Here, we used the term ‘chorioamnionitis’ for infants, who were born from U. urealyticum- or M. hominis-positive mothers or acute inflammatory change in the placental pathology. U. urealyticum and M. hominis are the most common organisms from infected amniotic fluid and placentas that contributes to adverse pregnancy outcomes including preterm birth and neonatal morbidities.8, 9, 17, 18, 19 Perinatal inflammation has been associated with adverse neurological effects in premature infants such as white matter injury, IVH, and cerebral palsy. Recently, a number of studies have assessed the relationship between maternal, placental infection/inflammation, and ROP.17, 20, 21 The role of perinatal infection/inflammation in ROP pathogenesis remains unclear, but several studies have reported positive association of chorioamnionitis with the occurrence and progression of ROP.8, 17, 20, 21 The production of proinflammatory cytokines, such as tumor necrosis factor-α, interleukins (IL)-1, IL-6, and IL-8 can be induced in the fetal brain by maternal intrauterine infection/inflammation, which in turn may attribute to the development of severe ROP, especially in the first 72 h of life.22 The lack of insulin-like growth factor (IGF-1) as a result of perinatal inflammation, an important non-oxygen regulated factor in ROP, is critical to the normal development of the retinal vasculature,23 may indicate a lack of vascular growth and subsequent proliferative ROP.24 Dammann et al21 suggested that extreme prematurity with multiple hits of perinatal inflammation appear to be involved in ROP etiology and progression. Our findings suggest that antenatal infection/inflammation itself may inhibit retinal vascular growth in utero and predispose infants toward rapidly-progressing ROP in zone I or posterior zone II. Other known risk factors25 such as IVH, NEC, BPD, PDA, sepsis, oxygen supplementation, maternal age, PROM, and preeclampsia were not considered meaningful in this study.
The present study investigated an association of thrombocytopenia with development of APROP. Thrombocytopenia is a common condition in newborns, especially those born with extremely low GA and BW.26 A ≥30% drop in platelet counts also without reaching thrombocytopenia as well as thrombocytopenia have been suggested to predict infantile morbidities and mortality.27 Several studies have indicated that platelets are important regulators of angiogenesis, by storing and transporting angiogenic regulatory proteins such as VEGF within platelet alpha granules.28, 29, 30 Therefore, thrombocytopenia may contribute to insufficient elimination of VEGF from the immature retina causing unregulated retinal neovascularization and subsequent development of ROP.31, 32 However, our study did not show any statistical association of thrombocytopenia and mean platelet count with development of APROP after adjusting for GA. This might be due to the single retrospective extraction of the platelet levels and the small sample size of our study. Further longitudinal data are required to clarify the exact relationship between low platelet counts and APROP.
Regarding to treatment outcome, laser treatment tended to be heavier in zone I APROP eyes than posterior zone II APROP eyes, not to speak of non-APROP eyes (Table 2). Disease severity and a more posterior position of the ridge, which result in an increased area of avascular retina requiring treatment, are the likely causes of more intensive therapy.6 Among infants with APROP, 18 eyes (85.7%) from the zone I APROP group and 22 eyes (81.5%) from the posterior zone II APROP group achieved favorable anatomical outcome, which was lower than the treatment-requiring non-APROP group, of which 143 eyes (95.3%) had a favorable outcome. Similar to previous studies, more favorable anatomical outcomes were achieved by laser monotherapy in infants with non-zone I ROP than in infants with zone I ROP.7, 33 Bevacizumab eliminates the angiogenic threat of ROP because of the role of VEGF in stimulating neovascularization in ROP.6 Kim et al34 reported prompt regression of plus sign and favorable outcome after combined zone I sparing laser photocoagulation and 0.25 mg/0.01 ml of intravitreal bevacizumab injection. In our study, 14 APROP eyes were treated with laser photocoagulation combined with intravitreal bevacizumab injection. Retreatment was required in two eyes, and favorable anatomical outcome was ultimately achieved in 12 eyes without retinal detachment. This overall high anatomical favorable outcome of APROP implies that careful screening and timely aggressive treatment can lead to good outcomes even in infants with APROP. Further investigation is required regarding long-term functional outcomes such as visual acuity and field, refractive error, and development of strabismus in APROP-treated infants.
Although our results should be interpreted with caution due to the small sample size and single-center nature of the study, it is noteworthy that not only low GA and BW at birth but also being small for GA and chorioamnionitis could be the predictors for developing APROP. Thus, the results of our study suggest that, ophthalmologists should always beware of the possibility of severe ROP development, especially in premature infants with antenatal maternal environmental risk factors that may interfere with normal in utero retinal vascular growth.

References
Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK . Childhood blindness. J AAPOS 1999; 3 (1): 26–32.
Palmer EA . Results of U.S. randomized clinical trial of cryotherapy for ROP (CRYO-ROP). Doc Ophthalmol 1990; 74 (3): 245–251.
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003; 121 (12): 1684–1694.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007; 196 (2): 147.e1–147.e8.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123 (7): 991–999.
Gunn DJ, Cartwright DW, Gole GA . Prevalence and outcomes of laser treatment of aggressive posterior retinopathy of prematurity. Clin Exp Ophthalmol 2014; 42 (5): 459–465.
O'Keefe M, Lanigan B, Long VW . Outcome of zone 1 retinopathy of prematurity. Acta Ophthalmol Scand 2003; 81 (6): 614–616.
Chen ML, Allred EN, Hecht JL, Onderdonk A, VanderVeen D, Wallace DK et al. Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci 2011; 52 (10): 7052–7058.
Woo SJ, Park KH, Jung HJ, Kim S, Choe G, Ahn J et al. Effects of maternal and placental inflammation on retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2012; 250 (6): 915–923.
Tita AT, Andrews WW . Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010; 37 (2): 339–354.
Park SH, Yum HR, Kim S, Lee YC . Retinopathy of prematurity in Korean infants with birthweight greater than 1500g. Br J Ophthalmol 2016; 100 (6): 834–838.
Bardin C, Zelkowitz P, Papageorgiou A . Outcome of small-for-gestational age and appropriate-for-gestational age infants born before 27 weeks of gestation. Pediatrics 1997; 100 (2): E4.
Gortner L, Wauer RR, Stock GJ, Reiter HL, Reiss I, Jorch G et al. Neonatal outcome in small for gestational age infants: do they really better? J Perinat Med 1999; 27 (6): 484–489.
Zhang S, Leske DA, Lanier WL, Holmes JM . Postnatal growth retardation exacerbates acidosis-induced retinopathy in the neonatal rat. Curr Eye Res 2001; 22 (2): 133–139.
Dhaliwal CA, Fleck BW, Wright E, Graham C, McIntosh N . Retinopathy of prematurity in small-for-gestational age infants compared with those of appropriate size for gestational age. Arch Dis Child Fetal Neonatal Ed 2009; 94 (3): F193–F195.
Misra A, Heckford E, Curley A, Allen L . Do current retinopathy of prematurity screening guidelines miss the early development of pre-threshold type 1 ROP in small for gestational age neonates? Eye 2008; 22 (6): 825–829.
Ozdemir R, Sari FN, Tunay ZO, Erdeve O, Canpolat FE, Oguz SS et al. The association between respiratory tract Ureaplasma urealyticum colonization and severe retinopathy of prematurity in preterm infants </=1250g. Eye 2012; 26 (7): 992–996.
Viscardi RM . Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed 2014; 99 (1): F87–F92.
Capoccia R, Greub G, Baud D . Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 2013; 26 (3): 231–240.
Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 2010; 67 (4): 394–400.
Dammann O, Brinkhaus MJ, Bartels DB, Dordelmann M, Dressler F, Kerk J et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev 2009; 85 (5): 325–329.
Silveira RC, Fortes Filho JB, Procianoy RS . Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Invest Ophthalmol Vis Sci 2011; 52 (3): 1297–1301.
Chen J, Smith LE . Retinopathy of prematurity. Angiogenesis 2007; 10 (2): 133–140.
Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA 2001; 98 (10): 5804–5808.
Port AD, Chan RV, Ostmo S, Choi D, Chiang MF . Risk factors for retinopathy of prematurity: insights from outlier infants. Graefes Arch Clin Exp Ophthalmol 2014; 252 (10): 1669–1677.
Christensen RD, Yoder BA, Baer VL, Snow GL, Butler A . Early-onset neutropenia in small-for-gestational-age infants. Pediatrics 2015; 136 (5): e1259–e1267.
Rastogi S, Olmez I, Bhutada A, Rastogi D . Drop in platelet counts in extremely preterm neonates and its association with clinical outcomes. J Pediatr Hematol Oncol 2011; 33 (8): 580–584.
Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008; 111 (3): 1227–1233.
Battinelli EM, Markens BA, Italiano JE Jr . Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 2011; 118 (5): 1359–1369.
Radziwon-Balicka A, Moncada de la Rosa C, Jurasz P . Platelet-associated angiogenesis regulating factors: a pharmacological perspective. Can J Physiol Pharmacol 2012; 90 (6): 679–688.
Vinekar A, Hegde K, Gilbert C, Braganza S, Pradeep M, Shetty R et al. Do platelets have a role in the pathogenesis of aggressive posterior retinopathy of prematurity? Retina 2010; 30 (4 Suppl): S20–S23.
Lundgren P, Lundberg L, Hellgren G, Holmstrom G, Hard AL, Smith LE et al. Aggressive posterior retinopathy of prematurity is associated with multiple infectious episodes and thrombocytopenia. Neonatology 2016; 111 (1): 79–85.
Kychenthal A, Dorta P, Katz X . Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina 2006; 26 (7 Suppl): S11–S15.
Kim J, Kim SJ, Chang YS, Park WS . Combined intravitreal bevacizumab injection and zone I sparing laser photocoagulation in patients with zone I retinopathy of prematurity. Retina 2014; 34 (1): 77–82.
Acknowledgements
We thank all the patients for participating in this study. This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning (NRF-2016R1C1B1016590).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahn, Y., Hong, K., Yum, H. et al. Characteristic clinical features associated with aggressive posterior retinopathy of prematurity. Eye 31, 924–930 (2017). https://doi.org/10.1038/eye.2017.18
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.18
This article is cited by
-
Blood neutrophil-to-lymphocyte ratio as a risk factor in treatment for retinopathy of prematurity
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Response to a letter to the editor related to Graefe’s Arch Clin Exp Ophthalmol. 2023 April; 261(4):951–957. “Blood neutrophil-to-lymphocyte ratio as a risk factor in the treatment of retinopathy of prematurity”
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Risk factors associated with Retinopathy of Prematurity development and progression
Scientific Reports (2022)
-
Retinopathy of prematurity: contribution of inflammatory and genetic factors
Molecular and Cellular Biochemistry (2022)
-
Aggressive posterior retinopathy of prematurity: a review on current understanding
Eye (2021)