Abstract
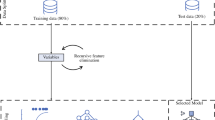
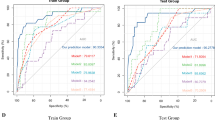
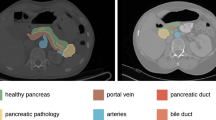
Clinically relevant postoperative pancreatic fistula (CR-POPF) is a life-threatening complication following pancreaticoduodenectomy (PD). Individualized preoperative risk assessment could improve clinical management and prevent or mitigate adverse outcomes. The aim of this study is to develop a machine learning risk model to predict occurrence of CR-POPF after PD from preoperative computed tomography (CT) scans. A total of 100 preoperative high-quality CT scans of consecutive patients who underwent pancreaticoduodenectomy in our institution between 2011 and 2019 were analyzed. Radiomic and morphological features extracted from CT scans related to pancreatic anatomy and patient characteristics were included as variables. These data were then assessed by a machine learning classifier to assess the risk of developing CR-POPF. Among the 100 patients evaluated, 20 had CR-POPF. The predictive model based on logistic regression demonstrated specificity of 0.824 (0.133) and sensitivity of 0.571 (0.337), with an AUC of 0.807 (0.155), PPV of 0.468 (0.310) and NPV of 0.890 (0.084). The performance of the model minimally decreased utilizing a random forest approach, with specificity of 0.914 (0.106), sensitivity of 0.424 (0.346), AUC of 0.749 (0.209), PPV of 0.502 (0.414) and NPV of 0.869 (0.076). Interestingly, using the same data, the model was also able to predict postoperative overall complications and a postoperative length of stay over the median with AUCs of 0.690 (0.209) and 0.709 (0.160), respectively. These findings suggest that preoperative CT scans evaluated by machine learning may provide a novel set of information to help clinicians choose a tailored therapeutic pathway in patients candidated to pancreatoduodenectomy.




Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability (software application or custom code)
Software code could be available from the corresponding author on reasonable request under written agreement.
References
Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM (2007) Clinical and economic validation of the international study group of pancreatic fistula (ISGPF) classification scheme. Ann Surg. https://doi.org/10.1097/01.sla.0000251708.70219.d2
Vollmer CM, Sanchez N, Gondek S et al (2012) A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. https://doi.org/10.1007/s11605-011-1753-x
Ahmad SA, Edwards MJ, Sutton JM et al (2012) Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. https://doi.org/10.1097/SLA.0b013e318265ef0b
Williamsson C, Ansari D, Andersson R, Tingstedt B (2017) Postoperative pancreatic fistula-impact on outcome, hospital cost and effects of centralization. HPB. https://doi.org/10.1016/j.hpb.2017.01.004
Fuks D, Piessen G, Huet E et al (2009) Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg 197(6):702–709. https://doi.org/10.1016/j.amjsurg.2008.03.004
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM (2013) A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 216(1):1–14. https://doi.org/10.1016/j.jamcollsurg.2012.09.002
Mungroop TH, Van Rijssen LB, Van Klaveren D et al (2019) Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg 269(5):937–943. https://doi.org/10.1097/SLA.0000000000002620
Chen JY, Feng J, Wang XQ, Cai SW, Dong JH, Chen YL (2015) Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol 21(19):5926–5933. https://doi.org/10.3748/wjg.v21.i19.5926
Kim JY, Park JS, Kim JK, Yoon DS (2013) A model for predicting pancreatic leakage after pancreaticoduodenectomy based on the international study group of pancreatic surgery classification. Korean J Hepato Biliary Pancreatic Surg 17(4):166. https://doi.org/10.14701/kjhbps.2013.17.4.166
Roberts KJ, Hodson J, Mehrzad H et al (2014) A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB 16(7):620–628. https://doi.org/10.1111/hpb.12186
Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T (2011) A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg 35(12):2747–2755. https://doi.org/10.1007/s00268-011-1253-x
Pratt WB, Callery MP, Vollmer CM (2008) Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 32(3):419–428. https://doi.org/10.1007/s00268-007-9388-5
Sandini M, Bernasconi DP, Fior D et al (2016) A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition 32(11–12):1231–1237. https://doi.org/10.1016/j.nut.2016.04.002
Pecorelli N, Carrara G, De Cobelli F et al (2016) Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. https://doi.org/10.1002/bjs.10063
Wellner UF, Kayser G, Lapshyn H et al (2010) A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB 12(10):696–702. https://doi.org/10.1111/j.1477-2574.2010.00239.x
Sandini M, Bernasconi DP, Ippolito D et al (2015) Preoperative computed tomography to predict and stratify the risk of severe pancreatic fistula after pancreatoduodenectomy. Med (US) 94(31):1–7. https://doi.org/10.1097/MD.0000000000001152
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Tempero MA, Malafa MP, Chiorean EG et al (2019) Pancreatic adenocarcinoma, version 1.2019 featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw. 17(3):203–210. https://doi.org/10.6004/jnccn.2019.0014
Dindo D, Demartines N, Clavien P (2004) Classification of Surgical Complications. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surg (US) 161(3):584–591. https://doi.org/10.1016/j.surg.2016.11.014
Pecorelli N, Carrara G, De Cobelli F et al (2016) Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg 103(4):434–442. https://doi.org/10.1002/bjs.10063
Haralick RM, Dinstein I, Shanmugam K (1973) Textural features for image classification. IEEE Trans Syst Man Cybern SMC-3(6):610–621. https://doi.org/10.1109/TSMC.1973.4309314
Xu DH, Kurani AS, Furst JD, Raicu DS (2004) Run-length encoding for volumetric texture. Image Process Proc Fourth IASTED Int Conf Vis Imaging 27:534–539
Amadasun M, King R (1989) Texural features corresponding to texural properties. IEEE Trans Syst Man Cybern. https://doi.org/10.1109/21.44046
Thibault G, Fertil B, Navarro C et al (2014) Texture indexes and gray level size zone matrix application to cell nuclei classification. Pattern Recognit Inf Process 2009:140–145
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85(1):115–122. https://doi.org/10.1152/jappl.1998.85.1.115
Van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-17-0339
Bergstra J, Bengio Y (2012) Random search for hyper-parameter optimization. J Mach Learn Res 13:281–305
Tibshirani R (1996) Regression shrinkage and selection via the lasso. J R Stat Soc Ser B. https://doi.org/10.1111/j.2517-6161.1996.tb02080.x
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP (2002) SMOTE: synthetic minority over-sampling technique. J Artif Intell Res. https://doi.org/10.1613/jair.953
Shen W, Punyanitya M, Wang ZM et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00744.2004
Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. https://doi.org/10.1139/H08-075
Bihorac A, Ozrazgat-Baslanti T, Ebadi A et al (2019) MySurgeryRisk: development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg 269(4):652–662. https://doi.org/10.1097/SLA.0000000000002706
Merath K, Hyer JM, Mehta R et al (2020) Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. https://doi.org/10.1007/s11605-019-04338-2
Kambakamba P, Mannil M, Herrera PE et al (2020) The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: a proof-of-principle study. Surg (US) 167(2):448–454. https://doi.org/10.1016/j.surg.2019.09.019
Han IW, Cho K, Ryu Y et al (2020) Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence. World J Gastroenterol. https://doi.org/10.3748/WJG.V26.I30.4453
Funding
No funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study concept and design, data interpretation, drafting, final approval, and accountability for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional Ethics Committee of Humanitas Clinical and Research Center-IRCCS, Milan.
Consent to participate
For this type of study, formal consent is not required.
Consent for publication
Not applicable.
Research involving human participants and/or animals
The study was approved by the institutional research committee. This article does not contain any studies on animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Capretti, G., Bonifacio, C., De Palma, C. et al. A machine learning risk model based on preoperative computed tomography scan to predict postoperative outcomes after pancreatoduodenectomy. Updates Surg 74, 235–243 (2022). https://doi.org/10.1007/s13304-021-01174-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01174-5