Abstract
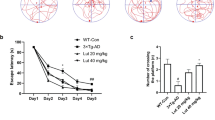
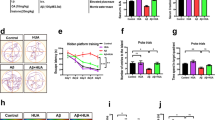
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases and is currently incurable. Amyloid β protein (Aβ) deposition is the main pathogenesis of AD, and many studies have shown that Aβ accumulation is toxic to neurons, leading to the inflammatory reaction, neuronal apoptosis, and neurofibrillary tangles. Thus, reducing Aβ levels might be a potential therapeutic strategy for AD. Liquiritigenin (LG), a dihydroflavone monomer compound extracted from natural plant licorice, has a variety of biological activities such as antioxidant, anti-tumor, anti-inflammatory and anti-virus. However, the exact function of LG in the pathogenesis of AD is elusive. Here, we reported that LG could significantly attenuate neuronal apoptosis in Aβ-induced N2A cells and APP/PS1 transgenic mice. Our in vivo and in vitro studies revealed that LG could alleviate the inflammation response, reflected by the reduction of NLRP3 and cleaved caspase-1. Meanwhile, we also found that LG was able to shift M1 type microglia towards M2 type microglia in Aβ-induced BV2 cells and AD mice. Furthermore, LG could reduce the Aβ levels by decreasing APP processing and accelerating Aβ clearance in AD mice. More importantly, daily treatment of LG (30 mg/kg day) for 90 days dramatically ameliorated the spatial learning and memory of AD mice. Taken together, these results suggest that LG can reduce the Aβ levels by regulating the M1/M2 transformation of microglia, thereby reversing memory decline during AD development, suggesting that LG may be a potential therapeutic agent for treating AD.






Similar content being viewed by others
References
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R (2017) Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 541(7636):217–221
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, Fowler C, Li QX, Martins R, Rowe C et al (2018) High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554(7691):249–254
Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422(6930):438–441
Bohm C, Chen F, Sevalle J, Qamar S, Dodd R, Li Y, Schmitt-Ulms G, Fraser PE, St George-Hyslop PH (2015) Current and future implications of basic and translational research on amyloid-beta peptide production and removal pathways. Mol Cell Neurosci 66(Pt A):3–11
Tan JZA, Gleeson PA (2019) The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer’s disease. Biochim Biophys Acta Biomembr 1861(4):697–712
Jang JY, Rhim H, Kang S (2018) NABi, a novel beta-sheet breaker, inhibits Abeta aggregation and neuronal toxicity: therapeutic implications for Alzheimer’s disease. Biochim Biophys Acta, Gen Subj 1862(1):71–80
Kong Y, Ruan L, Qian L, Liu X, Le Y (2010) Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme. J Neurosci 30(35):11848–11857
Gogoleva VS, Drutskaya MS, Atretkhany KS (2019) The role of microglia in the homeostasis of the central nervous system and neuroinflammation. Mol Biol 53(5):790–798
Hurtley SM (2017) A microglia type associated with AD. Science 357(6347):160–161
Liu FQ, Gao Q, Wang DD, Zhang ZX (2018) Effects of GBE50 on LPS/ATP induced NLRP3 inflammasome activation in primary rat microglia. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi. China J Chin Materia Medica 43(16):3346–3352
Garcez ML, Mina F, Bellettini-Santos T, da Luz AP, Schiavo GL, Macieski JMC, Medeiros EB, Marques AO, Magnus NQ, Budni J (2019) The involvement of NLRP3 on the effects of minocycline in an AD-like pathology induced by beta-amyloid oligomers administered to mice. Mol Neurobiol 56(4):2606–2617
Chan EWL, Krishnansamy S, Wong C, Gan SY (2019) The NLRP3 inflammasome is involved in the neuroprotective mechanism of neural stem cells against microglia-mediated toxicity in SH-SY5Y cells via the attenuation of tau hyperphosphorylation and amyloidogenesis. Neurotoxicology 70:91–98
Fan Z, Liang Z, Yang H, Pan Y, Zheng Y, Wang X (2017) Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J Neuroinflammation 14(1):256
Sierra-Filardi E, Puig-Kroger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, Bernabeu C, Vega MA, Corbi AL (2011) Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 117(19):5092–5101
Yousefi N, Sotoodehnejadnematalahi F, Heshmati-Fakhr N, Sayyah M, Hoseini M, Ghassemi S, Aliakbari S, Pourbadie HG (2019) Prestimulation of microglia through TLR4 pathway promotes interferon beta expression in a rat model of Alzheimer’s disease. J Molec Neurosci 67(4):495–503
Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, Huang JY, Zhao XJ, Sun XL (2018) Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell 17(4):e12774
Zhu X, Shi J, Li H (2018) Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-kappaB and NLRP3 inflammasome pathways. Biomed Pharmacotherap 106:976–982
Ramalingam M, Kim H, Lee Y, Lee YI (2018) Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from Radix Glycyrrhizae in human health and disease models. Front Aging Neurosci 10:348
Jo DS, Shin DW, Park SJ, Bae JE, Kim JB, Park NY, Kim JS, Oh JS, Shin JW, Cho DH (2016a) Attenuation of Abeta toxicity by promotion of mitochondrial fusion in neuroblastoma cells by liquiritigenin. Arch Pharm Res 39(8):1137–1143
Hongyan L, Suling W, Weina Z, Yajie Z, Jie R (2016) Antihyperuricemic effect of liquiritigenin in potassium oxonate-induced hyperuricemic rats. Biomed Pharmacotherap 84:1930–1936
Liu RT, Zou LB, Lu QJ (2009) Liquiritigenin inhibits Abeta(25-35)-induced neurotoxicity and secretion of Abeta(1-40) in rat hippocampal neurons. Acta Pharmacol Sin 30(7):899–906
Theriault P, ElAli A, Rivest S (2016) High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 7(42):67808–67827
Katoh R, Bray CE, Suzuki K, Komiyama A, Hemmi A, Kawaoi A, Oyama T, Sugai T, Sasou S (1995) Growth activity in hyperplastic and neoplastic human thyroid determined by an immunohistochemical staining procedure using monoclonal antibody MIB-1. Hum Pathol 26(2):139–146
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297(5868):681–683
Liang H, Salinas RA, Leal BZ, Kosakowska-Cholody T, Michejda CJ, Waters SJ, Herman TS, Woynarowski JM, Woynarowska BA (2004) Caspase-mediated apoptosis and caspase-independent cell death induced by irofulven in prostate cancer cells. Mol Cancer Ther 3(11):1385–1396
Hou Z, Li F, Chen J, Liu Y, He C, Wang M, Mei T, Zhang Y, Song L, Shao X (2019) Beneficial effects of sagacious Confucius’ pillow elixir on cognitive function in senescence-accelerated P8 mice (SAMP8) via the NLRP3/caspase-1 pathway. Evid-Based Complement Altern med: eCAM 2019:3097923
Hung COY, Livesey FJ (2018) Altered gamma-secretase processing of APP disrupts lysosome and autophagosome function in monogenic Alzheimer’s disease. Cell Rep 25(13):3647–3660 e3642
Li S, Jin M, Liu L, Dang Y, Ostaszewski BL, Selkoe DJ (2018) Decoding the synaptic dysfunction of bioactive human AD brain soluble Abeta to inspire novel therapeutic avenues for Alzheimer’s disease. Acta Neuropathologic Commu 6(1):121
van der Kant R, Goldstein LSB, Ossenkoppele R (2019) Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A et al (2019) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet 51(3):414–430
Jo DS, Shin DW, Park SJ, Bae JE, Kim JB, Park NY, Kim JS, Oh JS, Shin JW, Cho DH (2016b) Erratum to: Attenuation of Abeta toxicity by promotion of mitochondrial fusion in neuroblastoma cells by liquiritigenin. Arch Pharm Res 39(9):1337
Kimura A, Hata S, Suzuki T (2016) Alternative selection of beta-site APP-cleaving enzyme 1 (BACE1) cleavage sites in amyloid beta-protein precursor (APP) harboring protective and pathogenic mutations within the Abeta sequence. J Biol Chem 291(46):24041–24053
Thawkar BS, Kaur G (2019) Inhibitors of NF-kappaB and P2X7/NLRP3/Caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J Neuroimmunol 326:62–74
Li Q, Liu D, Pan F, Ho CSH, Ho RCM (2019) Ethanol exposure induces microglia activation and neuroinflammation through TLR4 activation and SENP6 modulation in the adolescent rat hippocampus. Neural Plasticity 2019:1648736
Yin J, Zhao F, Chojnacki JE, Fulp J, Klein WL, Zhang S, Zhu X (2018) NLRP3 Inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol Neurobiol 55(3):1977–1987
Tejera D, Mercan D, Sanchez-Caro JM, Hanan M, Greenberg D, Soreq H, Latz E, Golenbock D, Heneka MT (2019) Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J 38(17):e101064
Allendorf DH, Puigdellivol M, Brown GC (2019) Activated microglia desialylate their surface, stimulating complement receptor 3-mediated phagocytosis of neurons. Glia
Gupta N, Shyamasundar S, Patnala R, Karthikeyan A, Arumugam TV, Ling EA, Dheen ST (2018) Recent progress in therapeutic strategies for microglia-mediated neuroinflammation in neuropathologies. Expert Opin Ther Targets 22(9):765–781
Long J, Wang Q, He H, Sui X, Lin G, Wang S, Yang J, You P, Luo Y, Wang Y (2019) NLRP3 inflammasome activation is involved in trimethyltin-induced neuroinflammation. Brain Res 1718:186–193
Liu Y, Zeng R, Wang Y, Huang W, Hu B, Zhu G, Zhang R, Li F, Han J, Li Y (2019) Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res 1724:146422
Acknowledgments
The authors thank Professor Song for sharing the antibody.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81671257) and Natural Science Foundation of Chongqing (No. cstc2019jcyj-bshX0016).
Author information
Authors and Affiliations
Contributions
YD and ML performed the research. GH designed the research study and contributed essential reagents or tools. YD, KW, and GH analyzed the data. YD, YD, and GH wrote the manuscript. QY and MX supported several experiments, acquisition of data, analysis, and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 130 kb)
Rights and permissions
About this article
Cite this article
Du, Y., Luo, M., Du, Y. et al. Liquiritigenin Decreases Aβ Levels and Ameliorates Cognitive Decline by Regulating Microglia M1/M2 Transformation in AD Mice. Neurotox Res 39, 349–358 (2021). https://doi.org/10.1007/s12640-020-00284-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00284-z