Abstract
Objective
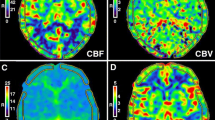
To explore the effort of cerebral small vessel disease (CSVD) on regional cerebral perfusion in patients with mild cognitive impairment (MCI) using NeuroGam™ software and evaluate the capability of brain perfusion single photon emission computed tomography (SPECT) in distinguishing MCI with and without CSVD.
Methods
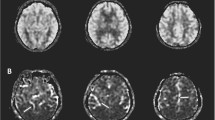
34 amnestic MCI subjects entered this study, conducting neuropsychological tests, MRI and 99mTechnetium ethyl cystine dimer brain perfusion SPECT imaging. All subjects were divided into those with CSVD and those without CSVD. Perfusion value was measured with Brodmann area (BA) mapping in these two groups. Automated software (NeuroGam™) was used for semi-quantitative analyses of perfusion value and comparison with normal database.
Results
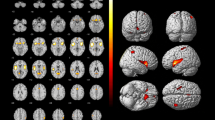
Compared with normal database, perfusion levels in BAs 23-left, 28 and 36-left of MCI without CSVD group had great deviations, while perfusion levels in BAs 21, 23, 24, 25, 28, 36, 38 and 47-left of MCI with CSVD group had great deviations. Furthermore, compared with CSVD group, there was significantly lower perfusion value in BA 7-left (P < 0.001) in MCI without CSVD group.
Conclusions
CSVD could interact with pathological changes related to AD, exacerbating hypoperfusion in BAs 21, 23, 28, 36, 38 while compensating for cerebral blood perfusion disorder in BA 7-left in MCI patients. Meanwhile, MCI patients with CSVD shared similar hypoperfusion with vascular cognitive impairment (VCI) in BAs 24, 25 and 47L. Brain perfusion SPECT may help improve our ability to differentiate MCI with and without CSVD.


Similar content being viewed by others
References
Du J, Zhu H, Zhou J, Lu P, Qiu Y, Yu L, et al. Structural brain network disruption at preclinical stage of cognitive impairment due to cerebral small vessel disease. Neuroscience. 2020;449:99–115.
Li K, Fu Z, Luo X, Zeng Q, Huang P, Zhang M, et al. The influence of cerebral small vessel disease on static and dynamic functional network connectivity in subjects Along Alzheimer’s disease continuum. Brain Connect. 2021;11(3):189–200.
Pantoni L, Marzi C, Poggesi A, Giorgio A, De Stefano N, Mascalchi M, et al. Fractal dimension of cerebral white matter: a consistent feature for prediction of the cognitive performance in patients with small vessel disease and mild cognitive impairment. Neuroimage Clin. 2019;24: 101990.
Lee MJ, Seo SW, Na DL, Kim C, Park JH, Kim GH, et al. Synergistic effects of ischemia and β-amyloid burden on cognitive decline in patients with subcortical vascular mild cognitive impairment. JAMA Psychiat. 2014;71(4):412–22.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.
Liu R, Chen H, Qin R, Gu Y, Chen X, Zou J, et al. The altered reconfiguration pattern of brain modular architecture regulates cognitive function in cerebral small vessel disease. Front Neurol. 2019;10:324.
Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13(Suppl 2):31–6.
Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83(14):1228–34.
Valotassiou V, Papatriantafyllou J, Sifakis N, Tzavara C, Tsougos I, Kapsalaki E, et al. Perfusion SPECT studies with mapping of Brodmann areas in differentiating Alzheimer’s disease from frontotemporal degeneration syndromes. Nucl Med Commun. 2012;33(12):1267–76.
Habert MO, Horn JF, Sarazin M, Lotterie JA, Puel M, Onen F, et al. Brain perfusion SPECT with an automated quantitative tool can identify prodromal Alzheimer’s disease among patients with mild cognitive impairment. Neurobiol Aging. 2011;32(1):15–23.
Valotassiou V, Papatriantafyllou J, Sifakis N, Tzavara C, Tsougos I, Psimadas D, et al. Clinical evaluation of brain perfusion SPECT with Brodmann areas mapping in early diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2015;47(3):773–85.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59.
Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87(4):375–83.
Zhou M, Zhang F, Zhao L, Qian J, Dong C. Entorhinal cortex: a good biomarker of mild cognitive impairment and mild Alzheimer’s disease. Rev Neurosci. 2016;27(2):185–95.
Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–43.
Johnson KA, Moran EK, Becker JA, Blacker D, Fischman AJ, Albert MS. Single photon emission computed tomography perfusion differences in mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(3):240–7.
Hirao K, Ohnishi T, Matsuda H, Nemoto K, Hirata Y, Yamashita F, et al. Functional interactions between entorhinal cortex and posterior cingulate cortex at the very early stage of Alzheimer’s disease using brain perfusion single-photon emission computed tomography. Nucl Med Commun. 2006;27(2):151–6.
Eichenbaum H, Schoenbaum G, Young B, Bunsey M. Functional organization of the hippocampal memory system. Proc Natl Acad Sci USA. 1996;93(24):13500–7.
Mosconi L, Pupi A, De Cristofaro MT, Fayyaz M, Sorbi S, Herholz K. Functional interactions of the entorhinal cortex: an 18F-FDG PET study on normal aging and Alzheimer’s disease. J Nucl Med. 2004;45(3):382–92.
Liu J, Zhang X, Yu C, Duan Y, Zhuo J, Cui Y, et al. Impaired parahippocampus connectivity in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2016;49(4):1051–64.
Grau-Olivares M, Arboix A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: a forerunner of vascular dementia? Expert Rev Neurother. 2009;9(8):1201–17.
Kim HJ, Park S, Cho H, Jang YK, San Lee J, Jang H, et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol. 2018;75(8):999–1007.
Wong FCC, Yatawara C, Low A, Foo H, Wong BYX, Lim L, et al. Cerebral small vessel disease influences hippocampal subfield atrophy in mild cognitive impairment. Transl Stroke Res. 2021;12(2):284–92.
Meguro K, Dodge HH. Vascular mild cognitive impairment: identifying disease in community-dwelling older adults, reducing risk factors, and providing support. The Osaki-Tajiri and Kurihara Projects. J Alzheimers Dis. 2019;70(s1):S293-s302.
Wang J, Liang Y, Chen H, Wang W, Wang Y, Liang Y, et al. Structural changes in white matter lesion patients and their correlation with cognitive impairment. Neuropsychiatr Dis Treat. 2019;15:1355–63.
Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015;9:23.
Zhou X, Hu X, Zhang C, Wang H, Zhu X, Xu L, et al. Aberrant functional connectivity and structural atrophy in subcortical vascular cognitive impairment: relationship with cognitive impairments. Front Aging Neurosci. 2016;8:14.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83.
Borroni B, Anchisi D, Paghera B, Vicini B, Kerrouche N, Garibotto V, et al. Combined 99mTc-ECD SPECT and neuropsychological studies in MCI for the assessment of conversion to AD. Neurobiol Aging. 2006;27(1):24–31.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38.
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701.
Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83(4):304–11.
Liu C, Zou L, Tang X, Zhu W, Zhang G, Qin Y, et al. Changes of white matter integrity and structural network connectivity in nondemented cerebral small-vessel disease. J Magn Reson Imaging. 2020;51(4):1162–9.
Chen W, Song X, Beyea S, D’Arcy R, Zhang Y, Rockwood K. Advances in perfusion magnetic resonance imaging in Alzheimer’s disease. Alzheimers Dement. 2011;7(2):185–96.
Jacobs KM. Brodmann’s areas of the cortex. New York: Springer; 2011.
Acknowledgements
This study was supported by Shanghai Science and Technology Commission Project: Molecular imaging study on mild cognitive impairment (No. 17411950102) and National Key Research and Development Plan Digital Diagnosis and Treatment Equipment Research and Development Project: Research and Practice of PET-CT Comprehensive Evaluation System and Training System (No. 2017YFC0113300).
Funding
Shanghai Science and Technology Commission Project: Molecular imaging study on mild cognitive impairment (No. 17411950102) and National Key Research and Development Plan Digital Diagnosis and Treatment Equipment Research and Development Project: Research and Practice of PET-CT Comprehensive Evaluation System and Training System (No. 2017YFC0113300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, Z., Lou, J., Wu, H. et al. Regional cerebral perfusion in patients with amnestic mild cognitive impairment: effect of cerebral small vessel disease. Ann Nucl Med 36, 43–51 (2022). https://doi.org/10.1007/s12149-021-01682-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01682-9