Abstract
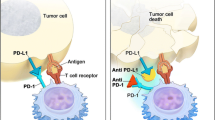
Bladder cancer is the most common malignancy involving the genitourinary system (Siegel et al. in CA Cancer J Clin, 66:7–30, 2016). In the USA, it is the fifth most common cancer and approximately 79,000 new cases will be diagnosed in 2017 (Siegel et al. in CA Cancer J Clin, 66:7–30, 2016). The mortality from bladder cancer is approximately 17,000 deaths each year (Siegel et al. in CA Cancer J Clin, 66:7–30, 2016). The incidence rate for bladder cancer is higher in men compared to women. Risk factors are predominantly related to tobacco smoking, although infection with Schistosoma haematobium is another risk factor in selected populations (Antoni et al. in Eur Urol, 71:96–108, 2017). Cisplatin-based systemic chemotherapy regimens remain the standard of care in both the neoadjuvant and metastatic setting for muscle-invasive bladder cancer (Gupta et al. in Cancer, 9(15):1–14, 2017; Von der Maase et al. in J Clin Oncol, 23:4602–4608, 2005; De Santis et al. in J Clin Oncol, 30:191–199, 2012; Bellmunt et al. in J Clin Oncol, 27: 4454–4461, 2009). There is an estimated overall survival of 9–15 months in metastatic bladder cancer in those who receive the standard of care platinum-based chemotherapy (Von der Maase et al. in J Clin Oncol, 23:4602–4608, 2005; De Santis et al. in J Clin Oncol, 30:191–199, 2012). The median survival, however, is significantly reduced after relapse in patient treated with platinum chemotherapy to less than 7 months (Bellmunt et al. in J Clin Oncol, 27: 4454–4461, 2009). Thus, this approach is preferred for patients who can tolerate this treatment as first-line chemotherapy (Gupta et al. in Cancer, 9(15):1–14, 2017). Until recently, there were few treatment options for those patients with poor performance status who are ineligible to receive cisplatin including renal insufficiency and multiple comorbidities or had disease progression after receiving platinum-based chemotherapy (Gupta et al. in Cancer, 9(15):1–14, 2017). With further understanding of tumor immune evasion, systemic immunotherapy which utilizes the patient’s own immune system directly to eradicate and target neoplastic cells, has now been approved for urothelial bladder cancer. Monoclonal antibodies that target programmed cell death protein 1 (PD-1), including Nivolumab and Pembrolizumab, and its ligand, PD-L1, including Atezolizumab, Durvalumab, Avelumab, have all been investigated and approved in the setting of metastatic refractory urothelial cancer (Gupta et al. in Cancer, 9(15):1–14, 2017; Von der Maase et al. in J Clin Oncol, 23:4602–4608, 2005; Zilchi et al. in BioMed Res Int, 2017, 2017, doi:10.1155/2017/5618174). Atezolizumab and Pembrolizumab have also been approved as first-line therapy in the setting of cisplatin-ineligible metastatic bladder cancer (Gupta et al. in Cancer, 9(15):1–14, 2017; Zilchi et al. in BioMed Res Int, 2017, 2017, doi:10.1155/2017/5618174). Those that target cytotoxic T-lymphocyte-associated protein 4, including Ipilimumab and Tremelimumab, have also been investigated and further studies are being performed (Gupta et al. in Cancer, 9(15):1–14, 2017; Zilchi et al. in BioMed Res Int, 2017, 2017, doi:10.1155/2017/5618174). This review outlines the systemic immunotherapies that have been approved or are currently being investigated.
Similar content being viewed by others
References
Finn O. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Supplement 8):viii6–9. doi:10.1093/annonc/mds256.
Pardoll D. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Rosenberg J, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20.
Massard C, Gordon M, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119–25.
Heery C, O’Sullivan-Coyne G, Madan R, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587–98.
Apolo A, Infante J, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117–24.
Patel M, Ellerton J, Infante J, et al. Avelumab in patients with metastatic urothelial carcinoma: pooled results from two cohorts of the phase 1b JAVELIN solid tumor trial. J Clin Oncol. 2017; 35(supplement 6S), abstract 330.
Sharma P, Callahan M, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (Check-Mate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–8.
Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (Check-Mate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22.
Plimack E, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212–20.
Balar A, Castellano D, O’Donnell P, et al. Pembrolizumab as first-line therapy in cisplatin-ineligible advanced urothelial cancer: results from the total KEYNOTE-052 study population. J Clin Oncol. 2017; 35(supplement 6S), abstract 284.
Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
Carthon B, Wolchok J, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16(10):2861–71.
Prescott S, Jackson A, Hawkyard S, et al. Mechanisms of action of intravesical bacille Calmette–Guérin: local immune mechanisms. Clin Infect Dis. 2000;31(Suppl 3):S91.
Grossman H, Natale R, Tangen C, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66.
Sternberg C, de Mulder P, Schornagel J, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19:2638–46.
Dash A, Pettus J, Herr H, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113:2471–7.
Advanced Bladder Cancer Meta-analysis Collaboration. Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:189–99 (discussion 199-201).
Siegel R, Miller K, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108.
Gupta S, Gill D, Poole A, Agarwal N. Systemic immunotherapy for urothelial cancer: current trends and future direction. Cancer. 2017;9(15):1–14.
Von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8.
De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9.
Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–61.
Zilchi C, Tucci M, Leone G, et al. Immunotherapy for Patients with Advanced Urothelial Cancer: Current Evidence and Future Perspectives. BioMed Res Int. 2017; 2017, Article ID 5618174. doi:10.1155/2017/5618174.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
No animal or human were involved in this study.
Rights and permissions
About this article
Cite this article
Katz, H., Wassie, E. & Alsharedi, M. Checkpoint inhibitors: the new treatment paradigm for urothelial bladder cancer. Med Oncol 34, 170 (2017). https://doi.org/10.1007/s12032-017-1029-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1029-8