Abstract
Objective
Metabolic syndrome is a chronic-metabolic disease caused by a variety of factors, including high peripheral blood insulin levels and insulin resistance. It has been reported that GLP-1 could regulate insulin resistance. It is not known whether and how GLP-1 protects from fat-induced inflammation and immune changes. We investigated if GLP-1 alters the populations of fat-induced inflammation and immune cells and the related mechanism.
Methods
We obtained obese C57BL/6J mice by feeding them high-fat food, then treated the obese mice with GLP-1+ high-fat diet (G + Hi), normal chow diet (Nor), or high-fat diet (Hi) (n = 20 for each group) for 8 weeks. The GLP-1 receptor−/− B6 group were fed with HFD for 8 weeks (GLP-1R KO + Hi). In vivo and in vitro experiments were conducted on mice immune cells to investigate the effects of GLP-1 on the changes of the immune components and functions in obesity.
Results
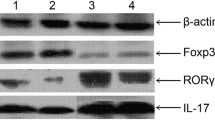
We found that GLP-1 could efficiently change the CD4+ T subsets and level of cytokines in high-fat-induced mice by GLP-1 receptor. Further, these changes were correlated with a reduction in fat content and serum lipid level. Interestingly, GLP-1 could enhance the function of Tregs in vitro.
Conclusion
Our data showed that GLP-1 has an important role in shaping the CD4+ T population in high-fat-diet-induced mice by GLP-1 receptor, possibly providing a new target for the treatment of metabolic syndrome.







Similar content being viewed by others
References
M.A. Nauck, J.J. Meier, Incretin hormones: their role in health and disease. Diabetes Obes. Metabol. 20(Suppl 1), 5–21 (2018)
S.K. Tasci, S.A. Bingol, GLP-1 localisation and proglucagon gene expression in healthy and diabetic mouse ileum. J. Vet. Res. 62(2), 237–242 (2018)
A. Itoh, J. Irie, H. Tagawa et al. GLP-1 receptor agonist, liraglutide, ameliorates hepatosteatosis induced by anti-CD3 antibody in female mice. J. Diabetes Complicat. 31(9), 1370–1375 (2017)
G.S. Carls, E. Tuttle, R.D. Tan et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care 40(11), 1469–1478 (2017)
E. Grasset, A. Puel, J. Charpentier et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metabol. 26(1), 278 (2017)
Q. Li, Y. Lin, S. Wang et al. GLP-1 inhibits high-glucose-induced oxidative injury of vascular endothelial Cells. Sci. Rep. 7(1), 8008 (2017)
J.P. Mayer, M.H. Tschop, R.D. DiMarchi, Once blind, now we see GLP-1 molecular action. Cell Metabol. 26(2), 289–291 (2017)
D.N. McBrayer, Y. Tal-Gan, Recent advances in GLP-1 receptor agonists for use in diabetes mellitus. Drug Dev. Res. 78(6), 292–299 (2017)
E. Adams, P. Genter, E. Keefe et al. The GLP-1 response to glucose does not mediate beta and alpha cell dysfunction in Hispanics with abnormal glucose metabolism. Diabetes Res Clin. Pract. 135, 185–191 (2018)
C.S. Bae, J. Song, The role of glucagon-like peptide 1 (GLP1) in type 3 diabetes: GLP-1 controls insulin resistance, neuroin-flammation and neurogenesis in the brain. Int. J. Mol. Sci. 18(11), 2493 (2017)
L.L. Baggio, J.R. Ussher, B.A. McLean et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metabol. 6(11), 1339–1349 (2017)
D.A. Briere, A.B. Bueno, E.J. Gunn et al. Mechanisms to elevate endogenous GLP-1 beyond injectable GLP-1 analogs and metabolic surgery. Diabetes 67(2), 309–320 (2018)
R. Caiazzo, J. Branche, M. Daoudi et al. Increased postprandial glucagon-like peptide-1 (GLP-1) production after endoscopic gastrointestinal bypass using the Cousin lumen-apposing stent in a porcine model. Endoscopy 50(1), 14–21 (2018)
M.A. Sanchez-Garrido, S.J. Brandt, C. Clemmensen et al. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia 60(10), 1851–1861 (2017)
U. Anyanwagu, U. Anyanwagu, J. Mamza, R. Donnelly, I. Idris, Effect of adding GLP-1RA on mortality, cardiovascular events, and metabolic outcomes among insulin-treated patients with type 2 diabetes: a large retrospective UK cohort study. Am. Heart J. 196, 18–27 (2018)
X. Cai, J. Li, M. Wang et al. GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int J. Med Sci. 14(12), 1203–1212 (2017)
P. Almgren, A. Lindqvist, U. Krus, et al. Genetic determinants of circulating GIP and GLP-1 concentrations. JCI Insight. 2(21), e93306 (2017)
D. Athauda, T. Foltynie, Protective effects of the GLP-1 mimetic exendin-4 in Parkinson’s disease. Neuropharmacology 136(Pt B), 260–270 (2018)
S. Baba, M. Iwasa, K. Higashi et al. Antidiabetic drug alogliptin protects the heart against ischemia-reperfusion injury through GLP-1 receptor-dependent and receptor-independent pathways involving nitric oxide production in rabbits. J. Cardiovasc. Pharmacol. 70(6), 382–389 (2017)
K. Coveleskie, L.A. Kilpatrick, A. Gupta et al. The effect of the GLP-1 analogue exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes. Sci. Pract. 3(4), 434–445 (2017)
H.M. Dorton, S. Luo, J.R. Monterosso, K.A. Page, Influences of dietary added sugar consumption on striatal food-cue reactivity and postprandial GLP-1 response. Front. Psychiatry 8, 297 (2017)
R. Garg, Nutritional insulin or Glp-1 receptor agonist: crossroads in the treatment of type 2 diabetes mellitus. Endocr. Pract. 23(11), 1357–1358 (2017)
C. Giezenaar, N.D. Luscombe-Marsh, A.T. Hutchison, et al., Effect of age on blood glucose and plasma insulin, glucagon, ghrelin, CCK, GIP, and GLP-1 responses to whey protein ingestion. Nutrients 10(1), 2 (2017)
A. Leonardini, R. D’Oria, M.A. Incalza et al. GLP-1 receptor activation inhibits palmitate-induced apoptosis via ceramide in human cardiac progenitor cells. J. Clin. Endocrinol. Metabol. 102(11), 4136–4147 (2017)
X. Wang, J. Liu, C. Li, et al., Impaired secretion of active GLP-1 in patients with hypertriglyceridaemia: a novel lipotoxicity paradigm? Diabetes Metabol. Res. Rev. 34(2), e2964 (2018)
Acknowledgements
This work was supported by grants from the Key Research and Development Program of Shandong Province (No. 2016GSF201012) and the Natural Science Foundation of Shandong Province (No. BS2015YY011) and the National Natural Science Foundation (No. 81500631).
Authors' contributions
LS conceived the project. SS and LS designed the experiments. SS analyzed the data and conducted experiments. SS wrote and edited the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sha, S., Liu, X., Zhao, R. et al. Effects of glucagon-like peptide-1 analog liraglutide on the systemic inflammation in high-fat-diet-induced mice. Endocrine 66, 494–502 (2019). https://doi.org/10.1007/s12020-019-02081-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02081-x