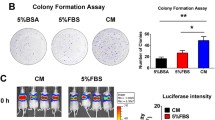
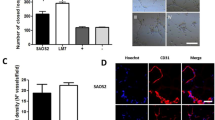
Abstract
During hematogenous metastasis, cancer cells escape from primary lesions and enter into the circulatory system, and only a few can colonize distant organs. However, the mechanism of cell survival and metastasis in the hematopoietic environment remains unclear. Angiorrhea is the character of pathological neovascularization in malignant tumors and commonly detected in osteosarcoma (OS), a bone tumor that prefers circulatory metastasis. In the present study, we focused on the notable role of serum albumin, the highest content in blood plasma, on OS progression. Our results indicated that serum albumin might act as a barrier against exogenous cancer cells during hematogenous metastasis. OS cells with high metastatic potential could gradually obtain strong viability through dedifferentiation under the effect of serum albumin in the angiorrhea region. Further exploration showed that serum albumin could increase the intracellular calcium concentration by activating the voltage-dependent calcium channel Cav2.1 in OS cells to affect the cytoskeleton, sequentially leading to dedifferentiation. Dedifferentiated OS cells with increased FAS apoptosis inhibitory molecule 2 (FAIM2) expression would gradually acquire survival ability, whereas knockdown of FAIM2 caused apoptosis in serum albumin. Moreover, FAIM2 overexpression rescued the viability of OS cells with low metastatic potential in serum albumin. In clinical specimens, OS cells showed markedly stronger positive staining of FAIM2 in the angiorrhea area. Taken together, our findings indicate that serum albumin in the angiorrhea region is a critical substance during pulmonary metastasis of OS cells. Angiorrhea is a nonnegligible prognostic element and FAIM2 might serve as a promising therapeutic target.








Similar content being viewed by others
References
Abarrategi A, Tornin J, Martinez-Cruzado L, Hamilton A, Martinez-Campos E, Rodrigo JP, González MV, Baldini N, Garcia-Castro J, Rodriguez R (2016) Osteosarcoma: cells-of-origin, cancer stem cells, and targeted therapies. Stem Cells Int 2016:3631764. https://doi.org/10.1155/2016/3631764
Aggerholm-Pedersen N, Maretty-Kongstad K, Keller J, Safwat A (2019) Serum biomarkers as prognostic factors for metastatic sarcoma. Clin Oncol (R Coll Radiol) 31:242–249
Anderson NL, Anderson NG (2002) The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1:845–867
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25
Botter SM, Neri D, Fuchs B (2014) Recent advances in osteosarcoma. Curr Opin Pharmacol 16:15–23
Butler TP, Gullino PM (1975) Quantitation of cell shedding into efferent blood of mammary. Cancer Res 35:512–516
Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL (2000) Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl Acad Sci USA 97:14608–14613
Dockal M, Carter DC, Ruker D (2000) Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J Biol Chem 275:3042–3050
Dolphin AC (2018) Voltage-gated calcium channels: their discovery, function and importance as drug targets. Brain Neurosci Adv 2:pii: 2398212818794805. https://doi.org/10.1177/2398212818794805
Evans TW (2002) Review article: Albumin as a drug—biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther 5:6–11
Farnsworth RH, Lackmann M, Achen MG, Stacker SA (2014) Vascular remodeling in cancer. Oncogene 33:3496–3505
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2:38–47
Hoagland LF 4th, Campa MJ, Gottlin EB, Herndon JE 2nd, Patz EF Jr (2007) Haptoglobin and posttranslational glycan-modified derivatives as serum biomarkers for the diagnosis of nonsmall cell lung cancer. Cancer 110:2260–2268
Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 7:653–664
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16:116–122
Lisak DA, Schacht T, Enders V, Habicht J, Kiviluoto S, Schneider J, Henke N, Bultynck G, Methner A (2015) The transmembrane Bax inhibitor motif (TMBIM) containing protein family: tissue expression, intracellular localization and effects on the ER CA2+-filling state. Biochim Biophys Acta 1853:2104–2114
Lu J, Song G, Tang Q, Zou C, Han F, Zhao Z, Yong B, Yin J, Xu H, Xie X, Kang T, Lam Y, Yang H, Shen J, Wang J (2015) IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J Clin Invest 125:1839–1856
Ma T, Zhang P, Hou Y, Ning H, Wang Z, Huang J, Gao M (2018) “Smart” nanoprobes for visualization of tumor microenvironments. Adv Healthc Mater 7:e1800391. https://doi.org/10.1002/adhm.201800391
Magazinovic V, Debijadi R, Pantelic D (1957) Comparison of effects of PVTD, Swedish dextran, blood and 0.9% NaCl in dogs in shock after hemorrhage. Vojnosanit Pregl 14:678–683
McMillan DC (2019) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39:534–540
Miao Y, Zhang H, Pan Y, Ren J, Ye M, Xia F, Huang R, Lin Z, Jiang S, Zhang Y, Songyang Z, Zhang Y (2017) Single-walled carbon nanotube: one specific inhibitor of cancer stem cells in osteosarcoma upon downregulation of the TGFβ1 signaling. Biomaterials 149:29–40
Mohseny AB, Machado I, Cai Y, Schaefer KL, Serra M, Hogendoorn PC, Llombart-Bosch A, Cleton-Jansen AM (2010) Functional characterization of osteosarcoma cell lines provides representative models to study the human disease. Lab Invest 91:1195–1205
Morvan MG, Lanier LL (2016) NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16:7–19
Pedersen T, Moller AM, Gotzsche PC (2005) Human albumin in critically ill patients. Crit Care Med 33:1183–1205
Peiris-Pagès M, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2015) Metastasis and oxidative stress: are antioxidants a metabolic driver of progression? Cell Metab 22:956–958
Phillips A, Shaper AG, Whincup PH (1989) Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 16:1434–1436
Planells-Ferrer L, Urresti J, Coccia E, Galenkamp KM, Calleja-Yagüe I, López-Soriano J, Carriba P, Barneda-Zahonero B, Segura MF, Comella JX (2016) Fas apoptosis inhibitory molecules: more than death-receptor antagonists in the nervous system. J Neurochem 139:11–21
Quan GM, Choong PF (2006) Anti-angiogenic therapy for osteosarcoma. Cancer Metastasis Rev 25:707–713
Raoufinia R, Mota A, Nozari S, Aghebati Maleki L, Balkani S, Abdolalizadeh J (2016) A methodological approach for purification and characterization of human serum albumin. J Immunoassay Immunochem 37:623–635
Rebecca LS, Kimberly DM, Ahmedin J (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Shao H, Wang A, Lauffenburger D, Wells A (2018) Tyro3-mediated phosphorylation of ACTN4 at tyrosines is FAK-dependent and decreases susceptibility to cleavage by m-Calpain. Int J Biochem Cell Biol 95:73–84
Strilic B, Offermanns S (2017) Intravascular survival and extravasation of tumor cells. Cancer Cell 32:282–293
Takeda Y, Shinzaki S, Okudo K, Moriwaki K, Murata K, Miyoshi E (2012) Fucosylated haptoglobin is a novel type of cancer biomarker linked to the prognosis after an operation in colorectal cancer. Cancer 118:3035–3043
Urresti J, Ruiz-Meana M, Coccia E, Arévalo JC, Castellano J, Fernández-Sanz C, Galenkamp KM, Planells-Ferrer L, Moubarak RS, Llecha-Cano N, Reix S, García-Dorado D, Barneda-Zahonero B, Comella JX (2016) Lifeguard inhibits fas ligand-mediated endoplasmic reticulum-calcium release mandatory for apoptosis in type II apoptotic cells. J Biol Chem 291:1221–1234
Vervliet T, Parys JB, Bultynck G (2016) Bcl-2 proteins and calcium signaling: complexity beneath the surface. Oncogene 35:5079–5092
Viallard C, Larrivée B (2017) Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20:409–426
Wirtz D, Konstantopoulos K, Searson PC (2011) The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11:512–522
Ye B, Cramer DW, Skates SJ, Gygi SP, Pratomo V, Fu L, Horick NK, Licklider LJ, Schorge JO, Berkowitz RS, Mok SC (2003) Haptoglobin-alpha subunit as potential serum biomarker in ovarian cancer: identification and characterization using proteomic profiling and mass spectrometry. Clin Cancer Res 9:2904–2911
Zhang H, Wu H, Zheng J, Yu P, Xu L, Jiang P, Gao J, Wang H, Zhang Y (2013) Transforming growth factor β1 signal is crucial for dedifferentiation of cancer cells to cancer stem cells in osteosarcoma. Stem Cells 31:433–446
Zhang Y, Pan Y, Xie C (2018) miR-34a exerts as a key regulator in the dedifferentiation of osteosarcoma via PAI-1–Sox2 axis. Cell Death Dis 9:777. https://doi.org/10.1002/stem.1298
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 31871413) and two Programs of Guangdong Science and Technology (2017B020230002 and 2016B030231001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All of the animal experiments were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals after approval from the Animal Ethics Committee of Sun Yat-sen University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Tetsuji Okamoto
Electronic supplementary material
Fig. S1.
SA-iOSCs representing characteristics of cancer stem cells. (a) IF of embryonic stem cells (ESCs) markers in adherent and SA-iOSCs MNNG/HOS cells. SSEA-1 is shown as red fluorescence and Sox2, CD133, and C-kit are shown as green fluorescence. Nuclei were stained with DAPI (Scale bar, 25 μm). (b) Micrographs of second and tenth passages of iOSCs induced by low-level serum albumin or TGF-β1 for 96 h (Scale bar, 100 μm). (c) Schematic diagram shows the differences in self-renewal ability between iOSCs induced by serum albumin or TGF-β1. (d) Detection of chemotherapeutic tolerance in adherent and SA-iOSCs MNNG/HOS cells (n = 5) under different concentrations of DOXO (0–400 ng/mL). P < 0.05=*. (JPG 804 kb)
Fig. S2.
Dedifferentiation process of MNNG/HOS cells was promoted by serum albumin. Representative microscopy images of MNNG/HOS cells cultured in serum-free medium supplemented with or without 0.5 mg/ml HSA or BSA (Scale bar, 100 μm). (JPG 979 kb)
Fig. S3.
Serum albumin did not affect OS cells through L-type VDCCs. (a) Cytotoxicity test of MNNG/HOS cells cultured with different concentrations of ω-conotoxin MVIIC, nitrendipine or nifedipine for 48 hours (n = 5). (b) Dedifferentiation of MNNG/HOS cells induced by serum albumin was not inhibited by 5 μM nitrendipine or 5 μM nifedipine treatment (Scale bar, 100 μm). (c) Cell death of U-2 OS cells induced by albumin was not inhibited by 5 μM nitrendipine or 5 μM nifedipine treatment (Scale bar, 100 μm). P < 0.05=*, P < 0.01=**, P < 0.001=***. (JPG 1324 kb)
Fig. S4.
The apoptosis induced by serum albumin in OS cells was mainly the extrinsic pathway of apoptosis. (a) qRT-PCR analysis of BCL2L1 mRNA expression level (n = 3) in MNNG/HOS cells and U-2 OS cells treated with gradient concentration of serum albumin for 96 h. (b) The expression levels of β-Actin, Bcl-xL, and cleaved caspase-8 in MNNG/HOS and U-2 OS cells were tested by WB after serum free, 5 mg/mL, and 50 mg/mL BSA treatment for 72 h. Representative images of WB are shown. P > 0.05= ns. (JPG 598 kb)
Fig. S5.
Knockdown or overexpression of FAIM2 in OS cells. mRNA levels (a, n = 3) and protein levels (b) of FAIM2 were downregulated by siRNA in MNNG/HOS cells. (c) TUNEL staining of MNNG/HOS-siRNA-NC and MNNG/HOS-siFAIM2 cells cultured in 50 mg/mL BSA for 48 h (Scale bar, 25 μm). The mRNA levels (d, n = 3) and protein levels (e) of FAIM2 were upregulated in U-2 OS cells. Representative images of WB are shown. (f) TUNEL staining of U2-pEGFP-N1 and U2-FAIM2 cells cultured in 50 mg/mL BSA for 48 h (Scale bar, 25 μm). (g) qRT-PCR analysis of FAIM2 expression in MNNG/HOS cells (n = 3). P < 0.01=**, P < 0.001=***. (JPG 1561 kb)
ESM 1
(DOCX 16 kb)
ESM 2
(PDF 375 kb)
ESM 3
(PDF 375 kb)
ESM4
(PDF 474 kb)
ESM 5
(PDF 938 kb)
ESM 6
(PDF 956 kb)
Rights and permissions
About this article
Cite this article
Pan, Y., Zhang, Y., Tang, W. et al. Interstitial serum albumin empowers osteosarcoma cells with FAIM2 transcription to obtain viability via dedifferentiation. In Vitro Cell.Dev.Biol.-Animal 56, 129–144 (2020). https://doi.org/10.1007/s11626-019-00421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00421-9