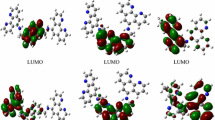
Abstract
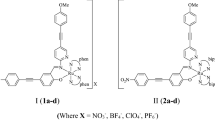
Five octahedral ruthenium(II) complexes with azoimine–quinoline (Azo) and α-diimine (L) ligands having the general formula [RuII(L)(Azo)Cl](PF6) (1–5) {Azo: PhN=NC(COMe)=NC9H6N, L = 4,4′-dimethoxy-2,2′-bipyridine (dmeb) (1), 4,4′-di-tertbutyl-2,2′-bipyridine (dtb) (2), 1,10-phenanthroline (phen) (3), 5-chlorophenanthroline (Clphen) (4), or 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen) (5)} were prepared by stepwise addition of the tridentate azoimine (H2Azo) and α-diimine (L) pro-ligands to RuCl3 in refluxing EtOH. The tridentate azoimine–quinoline ligands coordinate to ruthenium via the Azo-N′, N′-imine and N″-quinolone nitrogen atoms. The spectroscopic properties (IR, UV/Vis, 1H, 13C and 19F NMR) and electrochemical behavior of complexes 1–5 and the X-ray crystal structures of complexes 2 and 3 are presented. The coordination of Ru(II) to these strong π-acceptor ligands (Azo and L) results in a large anodic shift for the Ru(III/II) couples of 1.63–1.72 V versus NHE. The electronic spectra in MeCN and IR spectra in CH2Cl2 for complex 3 in its oxidized 3 + and reduced 3 − forms were investigated. The calculated absorption spectrum of 3 in MeCN was used to assign the UV–Vis absorption bands.












Similar content being viewed by others
References
Fahrni CJ, O’Halloran TV (1999) J Am Chem Soc 121:11448
Casida MK, Jamorski C, Casida KC, Salahub DR (1998) J Chem Phys 108:4439
Paul RC, Narula RC, Vasisht SK (1977) Transit Met Chem 2:152
Gustin VK, Sweet TR (1963) Anal Chem 35:44
Sutin N, Creutz C (1980) Pure Appl Chem 52:2717
Whitten DG (1980) Acc Chem Res 13:83
Al-Noaimi M, Crutchley RJ, AlDamen M, Rawashdeh A, Khanfar MA, Seppelt K (2011) Polyhedron 30:2075
Al-Noaimi M, El-khateeb M, Warad I, Haddad SF (2013) Inorg Chim Acta 400:20
Al-Noaimi M, Sunjuk M, El-khateeb M, Haddad SF, Haniyeh A, AlDamen M (2012) Polyhedron 42:66
Al-Noaimi M, El-khateeb M, Haddad S, Saadeh H (2010) Transit Met Chem 35:877
Al-Noaimi M, AlDamen MA (2012) Inorg Chim Acta 387:45
Al-Noaimi M, El-khateeb M, Haddad S, Sunjuk M, Crutchley RJ (2008) Polyhedron 27:3239
Al-Noaimi M, Abdel-Rahman OS, Fasfous II, El-khateeb M, Awwadi FF, Warad I (2014) Spectrochim Acta Part A Mol Biomol Spectrosc 125:375
Gennett T, Milner DF, Weaver MJ (1985) J Phys Chem 89:2787
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian Inc, Wallingford CT; GaussView5.0.9, Pittsburgh, Gaussian
Hay PJ, Wadt WR (1985) J Chem Phys 82:270
Wadt WR, Hay PJ (1985) J Chem Phys 82:284
Hay PJ, Wadt WR (1985) J Chem Phys 82:299
Andrae D, Haeussermann U, Dolg M, Stoll H, Preuss H (1990) Theor Chim Acc 77:123
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:454
Stratmann RE, Scuseria GE, Frisch MJ (1998) J Chem Phys 109:8218
Cossi M, Rega N, Scalmani G, Barone V (2003) Comput Chem 24:669
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839
CrysAlisPro, Agilent Technologies, Version 1.171.35.11 (Release 16–05–2011 CrysAlis171.NET) (Compiled May 16 2011,17:55:39)
Sheldrick G (2002) SHELXTL (XCIF, XL, XP, XPREP, XS), version 6.10. Bruker AXS Inc., Madison
Goswami S, Mukherjee R, Chakravorty A (1983) Inorg Chem 22:2825
Creutz C (1983) Prog Inorg Chem 30:1
Chen BH, Yao HH, Huang WT, Chattopadhyay P, Lo JM, Lu TH (1999) Solid State Sci 1:119
Gorelsky SI, Lever AB, Ebadi M (2002) Coord Chem Rev 230:97
Lever ABP (1985) Electronic absorption spectroscopy, 2nd edn. Elsevier Publishing Co., Amsterdam
Crutchley R, Lever B (1982) Inorg Chem 21:2276
Crutchley RJ, McCaw K, Lee FL, Gabe EJ (1990) Inorg Chem 29:2576
Lalrempuia R, Rao KM, Carroll PJ (2003) Polyhedron 22:605
Acknowledgments
M. Al-Noaimi would like to thank the Hashemite University (Jordan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Noaimi, M., Fasfous, I.I., Awwadi, F.F. et al. Ruthenium(II) complexes of azoimine and α-diimine ligands: synthesis, spectroscopic and electrochemical properties, crystal structures and DFT calculations. Transit Met Chem 41, 795–805 (2016). https://doi.org/10.1007/s11243-016-0080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0080-1