Abstract
Purpose
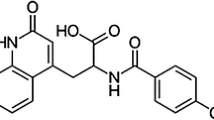
To prepare and thoroughly characterize a new polymorph of the broad-spectrum antibiotic minocycline from its hydrochloride dehydrate salts.
Methods
The new minocycline hydrochloride polymorph was prepared by means of the antisolvent effect caused by carbon dioxide. Minocycline recrystallized as a red crystalline hydrochloride salt, starting from solutions or suspensions containing CO2 and ethanol under defined conditions of temperature, pressure and composition.
Results
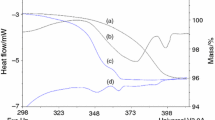
This novel polymorph (β-minocycline) revealed characteristic PXRD and FTIR patterns and a high melting point (of 247 ºC) compared to the initial minocycline hydrochloride hydrates (α-minocycline). Upon dissolution the new polymorph showed full anti-microbial activity. Solid-state NMR and DSC studies evidenced the higher chemical stability and crystalline homogeneity of β-minocycline compared to the commercial chlorohydrate powders. Molecular structures of both minocyclines present relevant differences as shown by multinuclear solid-state NMR.
Conclusions
This work describes a new crystalline structure of minocycline and evidences the ability of ethanol-CO2 system in removing water molecules from the crystalline structure of this API, at modest pressure, temperature and relatively short time (2 h), while controlling the crystal habit. This process has therefore the potential to become a consistent alternative towards the control of the solid form of APIs.












Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- ASAIS:
-
Atomization of supercritical antisolvent induced suspensions
- DSC:
-
Differential scanning calorimetry
- FTIR:
-
Fourier transform infrared spectroscopy
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- Nl:
-
Normal liter (mass of 1 l of gas at 1 atm and 20°C)
- PR-EOS:
-
Peng-Robinson equation of state
- PXRD:
-
Powder X-ray diffraction
- RH:
-
Relative humidity
- RVC:
-
Recrystallization visual cell
- SAS:
-
Supercritical antisolvent
- SELTICS:
-
Sideband elimination by temporary interruption of the chemical shift
- αMH:
-
Form α of minocycline hydrochloride
- βMH:
-
Form β of minocycline hydrochloride
References
Bishburg E, Bishburg K. Minocycline – an old drug for a new century: emphasis on methicillin-resistant Staphylococcus aureus (MRSA) and Acinetobacter baumannii. Int J Antimicrob Agents. 2009;34:395–401.
Huang V, Cheung CM, Kaatz GW, Rybak MJ. Evaluation of dalbavancin, tigecycline, minocycline, tetracycline, teicoplanin, and vancomycin against community-associated and multidrug-resistant hospital-associated methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2010;35:25–9.
Torrey EF, Davis JM. Adjunct treatments for schizophrenia and bipolar disorder: what to try when you are out of ideas. Clin Schizophr Relat Psychoses. 2012;5:208–16.
European Pharmacopoeia. 8th Ed. Strasbourg, France: Council of Europe; 2013. p. 2779.
Ahlneck C, Zografi G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int J Pharm. 1990;62:87–95.
Waterman KC, Adami RC. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int J Pharm. 2005;293:101–25.
Mendes Z, Antunes JR, Marto S, Heggie W. Crystalline minocycline base and processes for its preparation. WO2008102161-A2; US20100286417-A1.
Xiurong H, Jianming G, Linschen C. Minocycline hydrochloride hydrate crystal forms and preparation method thereof. CN101693669-A.
Morissette SL, Almarsson O, Peterson ML, Remenar JF, Read MJ, Lemmo AV, et al. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv Drug Deliv Rev. 2004;56:275–300.
Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9:2950–67.
Childs SL, Stahly GP, Park A. The salt-cocrystal continuum: the influence of crystal structure on ionization state. Mol Pharmaceutics. 2007;4:323–38.
Padrela L, Rodrigues MA, Velaga SP, Fernandes AC, Matos HA, Azevedo EG. Screening for pharmaceutical cocrystals using the supercritical fluid enhanced atomization process. J Supercrit Fluids. 2010;53:156–64.
Padrela L, Rodrigues MA, Velaga SP, Matos HA, Azevedo EG. Formation of indomethacin-saccharin cocrystals using supercritical fluid technology. Eur J Pharm Sci. 2009;38:9–17.
Rodrigues MA, Li J, Padrela L, Almeida AJ, Matos HA, Azevedo EG. Antisolvent effect in the production of lysozyme nano- and microparticles by supercritical fluid-assisted atomization processes. J Supercrit Fluids. 2009;48:253–60.
Tiago JM, Padrela L, Rodrigues MA, Matos HA, Almeida AJ, Azevedo EG. Single-step co-crystallization and lipid dispersion by supercritical enhanced atomization. Cryst Growth Des. 2013;13:4940–74.
Bettini R, Bonassi L, Castoro V, Rossi A, Zema L, Gazzaniga A, et al. Solubility and conversion of carbamazepine polymorphs in supercritical carbon dioxide. Eur J Pharm Sci. 2001;13:281–6.
Bettini R, Menabeni R, Tozzi R, Pranzo MB, Pasquali I, Chierotti MR, et al. Didanosine polymorphism in a supercritical antisolvent process. J Pharm Sci. 2010;99:1855–70.
Cocero MJ, Martin A, Mattea F, Varona S. Encapsulation and co-precipitation processes with supercritical fluids: fundamentals and applications. J Supercrit Fluids. 2009;47:546–55.
Martin A, Scholle K, Mattea F, Meterc D, Cocero MJ. Production of polymorphs of ibuprofen sodium by supercritical antisolvent (SAS) precipitation. Cryst Growth Des. 2009;9:2504–11.
Subra P, Laudani CG, Vega-Gonzalez A, Reverchon E. Precipitation and phase behavior of theophylline in solvent-supercritical CO2 mixtures. J Supercrit Fluids. 2005;35:95–105.
Reverchon E, Torino E, Dowy S, Braeuer A, Leipertz A. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization. Chem Eng J. 2010;156:446–58.
Cardoso MAT, Cabral JMS, Palavra AMF, Geraldes V. Characterization of minocycline powder micronized by a supercritical antisolvent (SAS) process. J Supercrit Fluids. 2008;47:247–58.
Li J, Rodrigues MA, Matos HA, Azevedo EG. VLE of carbon dioxide/ethanol/water: applications to volume expansion evaluation and water removal efficiency. Ind Eng Chem Res. 2005;44:6751–9.
Fischer K. A new method for the analytical determination of the water content of liquids and solids. Angew Chem. 1935;48:394–6.
Bruttel P, Schlink R. Water determination by Karl Fisher titration. Metrohm Ltd: Herisau, Switzerland; 2006.
Hong J, Harbison GS. Magic-Angle-spinning Side-band elimination by temporary interruption of the chemical-shift. J Magn Reson Ser A. 1993;105:128–36.
Stezwoski JJ. Chemical-structural properties of tetracycline derivatives. 1. Molecular structure and conformation of the free base derivatives. J Am Chem Soc. 1976;98:6012–8.
Curtis RD, Wasylishen RE. A nitrogen-15 nuclear magnetic resonance study of the tetracycline antibiotics. Can J Chem. 1991;69:834–8.
Mooibroek S, Wasylishen RE. A carbon-13 nuclear magnetic resonance study of solid tetracyclines. Can J Chem. 1987;65:357–62.
Levy GC, Lichter RL. Nitrogen-15 nuclear magnetic resonance spectroscopy. New York: John Wiley & Sons; 1979.
Casy AF, Yasin A. Application of 13C nuclear magnetic resonance spectroscopy to the analysis and structural investigation of tetracycline antibiotics and their common impurities. J Pharm Biomed Anal. 1984;2:19–36.
Casy AF, Yasin A. Stereochemical studies of tetracycline antibiotics and their common impurities by 400 MHz 1H NMR spectroscopy. Magn Reson Chem. 1985;23:767–70.
Mannargudi B, McNally D, Reynolds W, Uetrecht J. Bioactivation of minocycline to reactive intermediates by myeloperoxidase, horseradish peroxidase, and hepatic microsomes: implications for minocycline-induced lupus and hepatitis. Drug Metab Dispos. 2009;37:1806–18.
Mazzola EP, Melin JA, Wayland LG. 13C-NMR spectroscopy of three tetracycline antibiotics: minocycline hydrochloride, meclocycline, and rolitetracycline. J Pharm Sci. 1980;69:229–30.
Takeuchi Y, Imafuku Y, Nishikawa M. Reassignment of the 13C NMR spectrum of minomycin. Arkivoc. 2003; XV:2003:39–46.
Tatton AS, Pham TN, Vogt FG, Iuga D, Edwards AJ, Brown SP. Probing intermolecular interactions and nitrogen protonation in pharmaceuticals by novel 15 N-edited and 2D 14 N-1H solid-state NMR. Cryst Eng Comm. 2012;14:2654–9.
Tatton AS, Pham TN, Vogt FG, Iuga D, Edwards AJ, Brown SP. Probing hydrogen bonding in cocrystals and amorphous dispersions using 14 N-1H HMQC solid-state NMR. Mol Pharm. 2013;10:999–1007.
Bradley JP, Pickard CJ, Burley JC, Martin DR, Hughes LP, Cosgrove SD, et al. Probing intermolecular hydrogen bonding in sibenadet hydrochloride polymorphs by high-resolution 1H double-quantum solid-state NMR spectroscopy. J Pharm Sci. 2012;101:1821–30.
Baias M, Widdifield CM, Dumez J-N, Thompson HPG, Cooper TG, Salager E, et al. Powder crystallography of pharmaceutical materials by combined crystal structure prediction and solid-state 1H NMR spectroscopy. Phys Chem Chem Phys. 2013;15:8069–80.
Dudenko DV, Williams PA, Hughes CE, Antzutkin ON, Velaga SP, Brown SP, et al. Exploiting the synergy of powder X-ray diffraction and solid-state NMR spectroscopy in structure determination of organic molecular of solids. J Phys Chem C. 2013;117:12258–65.
Rodrigues MA, Padrela L, Matos HA, Azevedo EG, Pinheiro L, Bettencourt A, Castro ML, Almeida AJ, Duarte MA. New crystalline minocycline chlorohydrate, useful for preparing composite materials, prostheses, bone cements, implants, and controlled drug delivery formulations for the treatment of bacterial infections. WO2013095169-A1; PT106063-A1; 2013.
ACKNOWLEDGMENTS AND DISCLOSURES
We thank Dr. William Heggie and Dr. Zita Mendes from Hovione FarmaCiencia SA (Loures, Portugal) for the fruitful discussions and Professor Aida Duarte for the invaluable help at Microbiology Laboratory of FFUL (Lisbon, Portugal). For financial support, the authors are grateful to Fundação para a Ciência e Tecnologia (FCT), Lisbon (Grants SFRH/BD/39836/2007 and PTDC/EQUFTT/099912/2008, Strategic Project PEst-OE/SAU/UI4013/2011, project RECI/QEQ-QIN/0189/2012) and “Infra-estruturas de C&T” for the funding of the upgrade of the solid-state NMR spectrometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, M.A., Tiago, J.M., Padrela, L. et al. New Thermoresistant Polymorph from CO2 Recrystallization of Minocycline Hydrochloride. Pharm Res 31, 3136–3149 (2014). https://doi.org/10.1007/s11095-014-1406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1406-3