Abstract
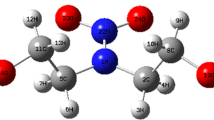
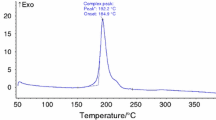
The isothermal decomposition of a new material with high energy, namely N-guanylurea dinitramide (FOX-12), was studied by a self-established isothermal decomposition gas metering device. The gas pressure versus time curves of the isothermal decomposition of FOX-12 were obtained at temperature intervals within 120 °C and 170 °C. An extremely long lag phase and acceleration period were found in the decomposition process of FOX-12. The kinetic parameters of FOX-12 were obtained by the Arrhenius equation and model-fitting method. Results of the two methods were consistent, and the activation energy of the acceleration period was smaller than that of the lag phase. With an average Ea of 159.4 kJ mol−1 and lnA of 34.74 s−1 were obtained at the lag phase, whereas an average Ea of 125.6 kJ mol−1 and lnA of 26.97 s−1 were noted at the acceleration period. The model-fitting method further proved that isothermal decomposition at 120–160 °C conformed to no. 28 corresponding to the reaction order n = 1/4, whether at the lag phase or acceleration period. The time required for decomposition was estimated to be 9.8 years when the extent of reaction reached 0.1% at ambient temperature (25 °C). The residual phase after FOX-12 decomposition was analyzed by Fourier transform infrared spectroscopy, and gases were analyzed by gas chromatography, and the possible decomposition process was proposed.









Similar content being viewed by others
References
Östmark H, Bemm U, Bergman H, Langlet A. N-guanylurea-dinitramide: a new energetic material with low sensitivity for propellants and explosives applications. Thermochim Acta. 2002;384(1–2):253–9.
Liu Q, Wang BZ, Zhang ZZ, Zhu CH, Lian P. Synthesis and properties of N-guanylurea dinitramide. Chin J Explos Propellants. 2006;29(1):29–31.
Liu HZ, Xu HX, Li Q, Li N, Wang XM, Chen JZ. Synthesis and application progress of GUDN. Chem Propellants Polym Mater. 2009;7(4):13–6.
Wang K, Chen JG, Wang BZ, Lu J, Wang WL, Liu FY, Zhou C, Lian P, Liu ZW, Liu ZT. Mechanisms and kinetics of the synthesis of FOX-12. Chem J Chin Univ. 2015;36(3):531–8.
Badgujar DM, Wagh RM, Pawar SJ, Sikder AK. Process optimization for synthesis of N-guanylurea dinitramide (GUDN). Propellants Explos Pyrot. 2014;39(5):658–61.
Liu N, Zhou C, Sun YJ, Wang BZ, Wang WL. Molecular dynamics simulations on crystal morphology of N-guanylurea-dinitramide. Chem J Chin Univ. 2017;38(12):2231–7.
Wang BZ, Liu Q, Zhang ZZ, Ji YP, Zhu CH. Study on properties of FOX-12. Chin J Energy Mater. 2004;12(1):38–9.
Zhang JQ, Zhao WW, Ji TZ, Gao HX, Hu RZ. The dissolution properties of N-guanylurea-dinitramide (FOX-12) in dimethyl sulfoxide (DMSO). J Therm Anal Calorim. 2014;115(1):641–6.
Perreault NN, Halasz A, Thiboutot S, Ampleman G, Hawari J. Joint photomicrobial process for the degradation of the insensitive munition, N-Guanylurea-dinitramide (FOX-12). Environ Sci Technol. 2013;47(10):5193–8.
Gao HX, Zhang H, Zhao FQ, Hu RZ, Ma HX. Kinetic behaviour of the exothermic decomposition reaction of N-guanylurea dinitramide. Acta Phys Chim Sin. 2008;24(3):453–8.
Santhosh G, Soumyamol PB, Sreejith M, Reshmi S. Iso-conversional approach for the non-isothermal decomposition kinetics of guanylurea dinitramide (GUDN). Thermochim Acta. 2016;632:46–51.
Zhao FQ, Chen P, Yuan HA, Gao SL, Hu RZ, Shi QZ. Thermochemical properties and non-isothermal decomposition reaction kinetics of N-guanylurea dinitramide (GUDN). Chin J Chem. 2004;22(2):136–41.
Chu SJ. Thermal analysis of explosives. 1st ed. Beijing: Science Press; 1994.
Egorshev VY, Sinditskii VP, Smirnov SP. A comparative study on two explosive acetone peroxides. Thermochim Acta. 2013;574:154–61.
Zeman S, Elbeih A, Yan QL. Note on the use of the vacuum stability test in the study of initiation reactivity of attractive cyclic nitramines in Formex P1 matrix. J Therm Anal Calorim. 2013;111(2):1503–6.
Liu R, Zhou ZN, Yin YL, Yang L, Zhong TL. Dynamic vacuum stability test method and investigation on vacuum thermal decomposition of HMX and CL-20. Thermochim Acta. 2012;537:13–9.
Liu R, Zhang TL, Yang L, Zhou ZN, Hu XC. Research on thermal decomposition of trinitrophloroglucinol salts by DSC, TG and DVST. Cent Eur J Chem. 2013;11(5):774–81.
Galwey AK. Thermal reactions involving solids: a personal view of selected features of decompositions, thermal analysis and heterogeneous catalysis. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09461-w.
Chovancová M, Zeman S. Study of initiation reactivity of some plastic explosives by vacuum stability test and non-isothermal differential thermal analysis. Thermochim Acta. 2007;460(1):67–76.
Xiao YY, Jin B, Peng RF, Zhang QC, Liu QQ, Chu SJ, Guo ZC. Thermal decomposition of CL-20 via a self-modified dynamic vacuum stability test. J Therm Anal Calorim. 2017;128(3):1833–40.
Luo LQ, Jin B, Xiao YY, Zhang QC, Chai ZH, Huang Q, Chu SJ, Peng RF. Study on the isothermal decomposition kinetics and mechanism of nitrocellulose. Polym Test. 2019;75:337–43.
Luo LQ, Guo PL, Jin B, Xiao YY, Zhang QC, Chu SJ, Peng RF. An improved isothermal decomposition dynamics research instrument and its application in HMX/TNT/Al composite explosive. J Therm Anal Calorim. 2019;139:2265–72.
Luo LQ, Jin B, Chai ZH, Huang Q, Chu SJ, Peng RF. Interaction and mechanism of nitrocellulose and N-methyl-4-nitroaniline by isothermal decomposition method. Cellulose. 2019;26:9021–33.
Huang Q, Liu XW, Xiao YY, Luo LQ, Luo G, Jin B, Peng RF, Chu SJ. Isothermal thermal decomposition of the HMX-based PBX explosive JOL-1. J Energy Mater. 2020;4:1–9.
Luo LQ, Chai ZH, Jin B, Huang Q, Guo ZL, Peng RF. Isothermal thermal decomposition of CL-20/HMX co-crystal explosive. CrystEngComm. 2020;22:1473–9.
Luo LQ, Jin B, Chai ZH, Huang Q, Chu SJ, Peng RF. The effects of aniline stabilizers on nitrocellulose based on isothermal thermal decomposition. Propellants Explos Pyrot. 2020;45:1–10.
Gábor V, Wang L, Skreiberg Y. Non-isothermal kinetics: best-fitting empirical models instead of model-free methods. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-09162-z.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and non-isothermal data. Thermochim Acta. 1999;340–341:53–68.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008.
Judge MD. An investigation of composite propellant accelerated ageing mechanisms and kinetics. Propellants Explos Pyrot. 2010;28(3):114–9.
Liu ZR, Shao YH, Ren XN, Chang H. Mathematical models and its calculations for predicting the life of explosives and propellants. Chin J Explos Propellants. 2016;39(2):1–7.
Acknowledgements
We are grateful for financial support by the Science Challenge Project (Project No. TZ2018004), the Natural Science Foundation of China (21875192), Key Projects of the Pre-research Fund of the General Armament Department (Project No. 6140720020101), National Defense Technology Foundation Project (Project No. JSJL2016404B002) and the Institute of Chemical Materials, China Academy of Engineering Physics (Project No. 18zh0080).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, Q., Jin, B., Guo, Z. et al. Isothermal decomposition and mechanism of N-guanylurea dinitramide. J Therm Anal Calorim 146, 2577–2585 (2021). https://doi.org/10.1007/s10973-020-10333-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10333-6