Abstract
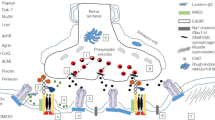
Congenital myasthenic syndromes (CMS) are genetic disorders due to mutations in genes encoding proteins involved in the neuromuscular junction structure and function. CMS usually present in young children, but perinatal and adult onset has been reported. Clinical presentation is highly heterogeneous, ranging from mild symptoms to severe manifestations, sometimes with life-threatening respiratory episodes, especially in the first decade of life. Although considered rare, CMS are probably underestimated due to diagnostic difficulties. Because of the several therapeutic opportunities, CMS should be always considered in the differential diagnosis of neuromuscular disorders. The Italian Network on CMS proposes here recommendations for proper CMS diagnosis and management, aiming to guide clinicians in their practical approach to CMS patients.


Similar content being viewed by others
References
Finlayson S, Beeson D, Palace J (2013) Congenital myasthenic syndromes: an update. Pract Neurol 13:80–91
Engel AG, Shen XM, Selcen D, Sine SM (2015) Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 14:461
Claeys KG, Maisonobe T, Bohm J, Laporte J, Hezode M, Romero NB et al (2010) Phenotype of a patient with recessive centronuclear myopathy and a novel BIN1 mutation. Neurology 74:519021
Robb SA, Sewry CA, Dowling JJ, Feng L, Cullup T, Lillis S et al (2011) Impaired neuromuscular transmission and response to aceylcholinesterase inhibitors in centronuclear myopathy. Neuromuscul Disord 21:379186
Liewluck T, Shen X-M, Milone M, Engel AG (2011) Endplate structure and parameters of neuromuscular transmission in sporadic centronuclear myopathy associated with myasthenia. Neuromuscul Disord 21:387195
Gibbs EM, Clarke NF, Rose K, Oates EC, Webster R, Feldman EL et al (2013) Neuromuscular junction abnrormalities in DNM2-related centronuclear myopathy. J Mol Med (Berl) 91:727337
Tsujino A, Maertens C, Ohno K, Shen XM, Fukuda T, Harper CM, Cannon SC, Engel AG (2003) Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci U S A 100:7377–7382
Arnold WD, Feldman DH, Ramirez S, He L, Kassar D, Quick A, Klassen TL, Lara M, Nguyen J, Kissel JT, Lossin C, Maselli RA (2015) Defective fast inactivation recovery of Nav 1.4 in congenital myasthenic syndrome. Ann Neurol 77:840–850
Belaya K, Rodriguez Cruz PM, Liu WW, Maxwell S, McGowan S, Farrugia ME et al (2015) Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain 138:2493–2504
Garg N, Yiannikas C, Hardy TA, Belaya K, Cheung J, Beeson D, Reddel SW (2016) Late presentations of congenital myasthenic syndromes: how many do we miss? Muscle Nerve 54:721–727
Parr JR, Andrew MJ, Finnis M, Beeson D, Vincent A, Jayawant S (2014) How common is childhood myasthenia? The UK incidence and prevalence of autoimmune and congenital myasthenia. Arch Dis Child 99:539–542
Natera-de Benito D, Töpf A, Vilchez JJ, González-Quereda L, Domínguez-Carral J, Díaz-Manera J, Ortez C, Bestué M, Gallano P, Dusl M, Abicht A, Müller JS, Senderek J, García-Ribes A, Muelas N, Evangelista T, Azuma Y, McMacken G, Paipa Merchan A, Rodríguez Cruz PM, Camacho A, Jiménez E, Miranda-Herrero MC, Santana-Artiles A, García-Campos O, Dominguez-Rubio R, Olivé M, Colomer J, Beeson D, Lochmüller H, Nascimento A (2017) Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscul Disord 27:1087–1098
Morar B, Gresham D, Angelicheva D, Tournev I, Gooding R, Guergueltcheva V, Schmidt C, Abicht A, Lochmüller H, Tordai A, Kalmár L, Nagy M, Karcagi V, Jeanpierre M, Herczegfalvi A, Beeson D, Venkataraman V, Warwick Carter K, Reeve J, de Pablo R, Kučinskas V, Kalaydjieva L (2004) Mutation history of the Roma/gypsies. Am J Hum Genet 75:596–609
Beeson D, Hantai D, Lochmuller H, Engel AG (2005) 126th International Workshop: congenital myasthenic syndromes, 24-26 September 2004, Naarden, the Netherlands. Neuromuscul Disord 15:498–512
Engel AG (2018) Congenital myasthenic syndromes in 2018. Curr Neurol Neurosci Rep 18:46
Abicht A, Dusl M, Gallenmuller C, Guergueltcheva V, Schara U, Della Marina A et al (2012) Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients. Hum Mutat 33:1474–1484
Eymard B, Stojkovic T, Sternberg D, Richard P, Nicole S, Fournier E, Béhin A, Laforêt P, Servais L, Romero N, Fardeau M, Hantaï D, Membres du réseau national Syndromes Myasthéniques Congénitaux (2013) Congenital myasthenic syndromes: difficulties in the diagnosis, course and prognosis, and therapy—the French National Congenital Myasthenic Syndrome Network experience. Rev Neurol (Paris) 169:S45–S55
Guergueltcheva V, Muller JS, Dusl M, Senderek J, Oldfors A, Lindbergh C et al (2012) Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations. J Neurol 259:838–850
Chaouch A, Muller JS, Guergueltcheva V, Dusl M, Schara U, Rakocevic-Stojanovic V et al (2012) A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol 259:474–481
Selcen D, Shen XM, Milone M, Brengman J, Ohno K, Deymeer F, Finkel R, Rowin J, Engel AG (2013) GFPT1-myasthenia: clinical, structural, and electrophysiologic heterogeneity. Neurology 81:370–378
Rodriguez Cruz PM, Belaya K, Basiri K, Sedghi M, Farrugia ME, Holton JL et al (2016) Clinical features of the myasthenic syndrome arising from mutations in GMPPB. J Neurol Neurosurg Psychiatry 87:802–809
Owen D, Topf A, Preethish-Kumar V, Lorenzoni PJ, Vroling B, scola RH et al (2018) Recessive variants of MuSK are associated with late onset CMS and predominant limb-girdle weakness. Am J Med Genet A 176:1594–1601
Hoffmann K, Muller JS, Stricker S, Megarbane A, Rajab A, Lindner TH et al (2006) Escobar syndrome is a prenatal myasthenia caused by disruption of the acetylcholine receptor fetal gamma subunit. Am J Hum Genet 79:303–312
Vogt J, Harrison BJ, Spearman H, Cossins J, Vermeer S, ten Cate LN, Morgan NV, Beeson D, Maher ER (2008) Mutation analysis of CHRNA1, CHRNB1, CHRND, and RAPSN genes in multiple pterygium syndrome/fetal akinesia patients. Am J Hum Genet 82:222–227
Vogt J, Morgan NV, Marton T, Maxwell S, Harrison BJ, Beeson D, Maher ER (2009) Germline mutation in DOK7 associated with fetal akinesia deformation sequence. J Med Genet 46:338–340
Michalk A, Stricker S, Becker J, Rupps R, Pantzar T, Miertus J, Botta G, Naretto VG, Janetzki C, Yaqoob N, Ott CE, Seelow D, Wieczorek D, Fiebig B, Wirth B, Hoopmann M, Walther M, Körber F, Blankenburg M, Mundlos S, Heller R, Hoffmann K (2008) Acetylcholine receptor pathway mutations explain various fetal akinesia deformation sequence disorders. Am J Hum Genet 82:464–476
Milone M, Shen XM, Selcen D, Ohno K, Brengman J, Iannacone ST et al (2009) Myasthenic syndrome due to defects in rapsyn: clinical and molecular findings in 39 patients. Neurology 73:228–235
Schara U, Christen HJ, Durmus H, Hietala M, Krabetz K, Rodolico C, Schreiber G, Topaloglu H, Talim B, Voss W, Pihko H, Abicht A, Müller JS, Lochmüller H (2010) Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur J Paediatr Neurol 14:326–333
Ben Ammar A, Petit F, Alexandri N, Gaudon K, Bauchè S, Rouche A et al (2010) Phenotype genotype analysis in 15 patients presenting a congenital myasthenic syndrome due to mutations in DOK7. J Neurol 257:754–766
Ohno K (2013) Glycosylation defects as an emerging novel cause leading to a limb-girdle type of congenital myasthenic syndromes. J Neurol Neurosurg Psychiatry 84:1064
Kinali M, Beeson D, Pitt MC, Jungbluth H, Simonds AK, Aloysius A, Cockerill H, Davis T, Palace J, Manzur AY, Jimenez-Mallebrera C, Sewry C, Muntoni F, Robb SA (2008) Congenital myasthenic syndromes in childhood: diagnostic and management challenges. J Neuroimmunol 201-202:6–12
Mihaylova V, Muller JS, Vilchez JJ, Salih MA, Kabiraj MM, D’Amico A et al (2008) Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain 131:747–759
Nicole S, Chaouch A, Torbergsen T, Bauche S, de Bruyckere E, Fontenille MJ et al (2014) Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain 137:2429–2443
McMacken G, Whittaker RG, Evangelista T, Abicht A, Dusl M, Lochmuller H (2018) Congenital myasthenic syndrome with episodic apnoea: clinical, neurophysiological and genetic features in the long-term follow-up of 19 patients. J Neurol 265:194–203
Robb SA, Muntoni F, Simonds AK (2010) Respiratory management of congenital myasthenic syndromes in childhood: workshop 8th December 2009, UCL Institute of Neurology, London, UK. Neuromuscul Disord 20:833–838
Palace J, Lashley D, Bailey S, Jayawant S, Carr A, McConville J, Robb S, Beeson D (2012) Clinical features in a series of fast channel congenital myasthenia syndrome. Neuromuscul Disord 22:112–117
Brueton LA, Huson SM, Cox PM, Shirley I, Thompson EM, Barnes PR et al (2000) Asymptomatic maternal myasthenia as a cause of the Pena-Shokeir phenotype. Am J Med Genet 92:1–6
Kosac A, Gavillet E, Whittaker RG (2013) Neurophysiological testing in congenital myasthenic syndromes: a systematic review of published normal data. Muscle Nerve 48:711–715
Benatar M (2006) A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord 16:459–467
Howard JF Jr (2013) Electrodiagnosis of disorders of neuromuscular transmission. Phys Med Rehabil Clin N Am 24:169–192
Nicole S, Azuma Y, Bauche S, Eymard B, Lochmuller H, Slater C (2017) Congenital myasthenic syndromes or inherited disorders of neuromuscular transmission: recent discoveries and open questions. J Neuromuscul Dis 4:269–284
Pascuzzi RM (2003) The edrophonium test. Semin Neurol 23:83–88
Finlayson S, Morrow JM, Rodriguez Cruz PM, Sinclair CD, Fischmann A, Thornton JS et al (2016) Muscle magnetic resonance in congenital myastenic syndromes. Muscle Nerve 54:211–219
Lindquist S, Stangel M (2011) Update on treatment options for Lambert–Eaton myasthenic syndrome: focus on use of amifampridine. Neuropsychiatr Dis Treat 7:341–349
Lashley D, Palace J, Jayawant S, Robb S, Beeson D (2010) Ephedrine treatment in congenital myasthenic syndrome due to mutations in DOK7. Neurology 74:1517–1523
Liewluck T, Selcen D, Engel AG (2011) Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and Dok-7 myasthenia. Muscle Nerve 44:789–794
Burke G, Hiscock A, Klein A, Niks EH, Main M, Manzur AY, Ng J, de Vile C, Muntoni F, Beeson D, Robb S (2013) Salbutamol benefits children with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disord 23:170–175
Rodriguez Cruz PM, Palace J, Ramjattan H, Jayawant S, Robb SA, Beeson D (2015) Salbutamol and ephedrine in the treatment of severe AChR deficiency syndromes. Neurology 85:1043–1047
Lee M, Beeson D, Palace J (2018) Therapeutic strategies for congenital myasthenic syndromes. Ann N Y Acad Sci 1412:129–136
Finlayson S, Spillane J, Kullmann DM, Howard R, Webster R, Palace J, Beeson D (2013) Slow channel congenital myasthenic syndrome responsive to a combination of fluoxetine and salbutamol. Muscle Nerve 47:279–282
Servais L, Baudoin H, Zehrouni K, Richard P, Sternberg D, Fournier E, Eymard B, Stojkovic T (2013) Pregnancy in congenital myasthenic syndrome. J Neurol 260:815–819
Esposito S, Bruno C, Berardinelli A, Filosto M, Mongini T, Morandi L, Musumeci O, Pegoraro E, Siciliano G, Tonin P, Marrosu G, Minetti C, Servida M, Fiorillo C, Conforti G, Scapolan S, Ansaldi F, Vianello A, Castaldi S, Principi N, Toscano A, Moggio M (2014) Vaccination recommendations for patients with neuromuscular disease. Vaccine 32:5893–5900
Ishigaki K, Nicolle D, Krejci E, Leroy JP, Koenig J, Fardeau M, Eymard B, Hantaı̈ D (2003) Two novel mutations in the COLQ gene cause endplate acetylcholinesterase deficiency. Neuromuscul Disord 13:236–244
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maggi, L., Bernasconi, P., D’Amico, A. et al. Italian recommendations for diagnosis and management of congenital myasthenic syndromes. Neurol Sci 40, 457–468 (2019). https://doi.org/10.1007/s10072-018-3682-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3682-x