Abstract
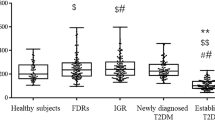
Amylin is the major constituent of pancreatic islet amyloid whose accumulation characterizes patients with type 2 diabetes mellitus (T2DM). Although amylin is tightly linked with T2DM, in many cases, proamylin may be the more toxic species. As the precursor of amylin, however, the pathophysiological role of proamylin remains unknown. In this study, we investigate whether serum levels of proamylin or amylin or the proamylin/amylin ratios are different among normal subjects and patients with impaired glucose regulation (IGR) and T2DM. Totally 79 subjects were divided into three groups according to the results of oral glucose tolerance test (OGTT); they were T2DM group (32 cases), IGR group (23cases), and normal glucose tolerance (NGT) group (24cases). Serum levels of amylin and proamylin were measured with an enzyme-linked immunosorbent assay (ELISA). The relationships between serum levels of proamylin, amylin, their ratios and anthropometric and metabolic parameters were also analyzed. The serum levels of proamylin were significantly higher in patients with IGR and T2DM than in control subjects. The serum levels of proamylin were significantly associated with IGR and T2DM, with the odds ratios of 1.589 (95%CI, 1.228–2.055, P < 0.01) and 1.860 (95%CI, 1.342–2.587, P < 0.01), respectively. Both fasting serum levels of proamylin and proamylin/amylin ratios were found to correlate negatively with HOMA-B and ΔI30/ΔG30. Serum levels of proamylin, amylin, and their ratios were positively correlated with HOMA-IR. BMI and HOMA-B were independent related factors with serum levels of proamylin. Our results suggest that proamylin may play an important role in amyloid deposit in patients with IGR and T2DM.
Similar content being viewed by others
References
Kahn SE (2003) The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19
Manco M, Giordano U, Turchetta A et al (2009) Insulin resistance and exercise capacity in male children and adolescents with non-alcholic fatty liver disease. Acta Diabetol 46(2):97–104
Butler AE, Janson J, Bonner-Weir S et al (2003) β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110
Ruchat SM, Elks CE, Loos RJ et al (2009) Association between insulin secretion, insulin sensitivity and type 2 diabetes susceptibility variants identified in genome-wide association studies. Acta Diabetol 46(3):217–226
Rhodes CJ (2005) Type 2 diabetes: a matter of beta-cell life and death? Science 307:380–384
Chistiakov DA, Potapov VA, Khodirev DC et al (2009) Genetic variations in the pancreatic ATP-sensitive potassium channel, beta-cell dysfunction, and susceptibility to type 2 diabetes. Acta Diabetol 46(1):43–49
Kahn SE, Andrikopoulos S, Verchere CB (1999) Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48:241–253
Clark A, Nilsson MR (2004) Islet amyloid: a complication of islet dysfunction or an aetiological factor in type 2 diabetes? Diabetologia 47:157–169
Federici M, Hribal M, Perego L et al (2001) High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 50(6):1290–1301
Chisalita SI, Lindström T, Eson Jennersjö P et al (2009) Differential lipid profile and hormonal response in type 2 diabetes by exogenous insulin aspart versus the insulin secretagogue repaglinide, at the same glycemic control. Acta Diabetol 46(1):35–42
Hartter E, Svoboda T, Ludvik B et al (1991) Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia 34:52–54
Kahn SE, D’Alessio DA, Schwartz MW et al (1990) Evidence of cosecretion of islet amyloid polypeptide and insulin by beta cells. Diabetes 39:634–638
Clark A, Charge SB, Badman MK et al (1996) Islet amyloid polypeptide: actions and role in the pathogenesis of diabetes. Biochem Soc Trans 24:594–599
Guardado-Mendoza R, Davalli AM, Chavez AO, et al (2009) Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci 18; 106(33):13992–13997
Huang CJ, Lin CY, Haataja L et al (2007) High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56(8):2016–2027
Butler AE, Janson J, Soeller WC et al (2003) Increased beta cell apoptosis prevents adaptive increase in beta cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52:2304–2314
Butler AE, Jang J, Gurlo T et al (2004) Diabetes due to a progressive defect in beta cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat): a new model for type 2 diabetes. Diabetes 53:1509–1516
Zraika S, Hull RL, Udayasankar J et al (2009) Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 52(4):626–635
Matveyenko AV, Gurlo T, Daval M et al (2009) Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes 58(4):906–916
Porte D Jr, Kahn SE (1989) Hyperproinsulinemia and amyloid in NIDDM: clues to etiology of islet β-cell dysfunction? Diabetes 38:1333–1336
Westermark P, Engstrom U, Westermark GT et al (1989) Islet amyloid polypeptide (IAPP) and pro-IAPP immunoreactivity in human islets of Langerhans. Diabetes Res Clin Pract 7:219–226
Hou X, Ling Z, Quartier E et al (1999) Prolonged exposure of pancreatic beta cells to raised glucose concentrations results in increased cellular content of islet amyloid polypeptide precursors. Diabetologia 42:188–194
Marzban L, Rhodes CJ, Steiner DF et al (2006) Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes 55(8):2192–2201
Wang J, Xu J, Finnerty J et al (2001) The prohormone convertase enzyme 2 (PC2) is essential for processing pro-islet amyloid polypeptide at the NH2-terminal cleavage site. Diabetes 50(3):534–539
Marzban L, Park K, Verchere CB (2003) Islet amyloid polypeptide and type 2 diabetes. Exp Gerontol 38:347–351
Krampert M, Bernhagen J, Schmucker J et al (2000) Amyloidogenicity of recombinant human proislet amyloid polypeptide (ProIAPP). Chem Biol 7:855–871
Paulsson JF, Andersson A, Westermark P et al (2006) Intracellular amyloid-like deposits contain unprocessed pro-islet amyloid polypeptide (proIAPP) in beta cells of transgenic mice overexpressing the gene for human IAPP and transplanted human islets. Diabetologia 49:1237–1246
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. 1. Diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabetes Med 15:539–553
Jha S, Sellin D, Seidel R, et al (2009) Amyloidogenic propensities and conformational properties of ProIAPP and IAPP in the presence of lipid bilayer membranes. J Mol Biol 26; 389(5):907–920
Hull RL, Westermark GT, Westermark P et al (2004) Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab 89:3629–3643
Alarcon C, Leahy JL, Schuppin GT et al (1995) Increased secretory demand rather than a defect in the proinsulin conversion mechanism causes hyperproinsulinemia in a glucose-infusion rat model of non-insulindependent diabetes mellitus. J Clin Invest 95:1032–1039
Janson J, Ashley RH, Harrison D et al (1999) The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediatesized toxic amyloid particles. Diabetes 48:491–498
Butler AE, Janson J, Soeller WC et al (2003) Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52:2304–2314
Demuro A, Mina E, Kayed R et al (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280:17294–17300
Skovronsky DM, Lee VM-Y, Trojanowski JQ (2006) Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol Mech Dis 1:151–170
Paulsson JF, Westermark GT (2005) Aberrant processing of human proislet amyloid polypeptide results in increased amyloid formation. Diabetes 54(7):2117–2125
Acknowledgments
This study was supported by Medical Science Research Fund of Chongqing Health Bureau (Number: 2008-2-96) and the Medical Science Research Fund of the First Affiliated Hospital, Chongqing Medical University. (Number: YXJ2009-03). We thank the Key Lab of Ophthalmology of The First Affiliated Hospital, Chongqing Medical University for the use of laboratory.
Conflict of interest statement
All authors declare no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, X., Ren, W., Zhang, S. et al. Serum levels of proamylin and amylin in normal subjects and patients with impaired glucose regulation and type 2 diabetes mellitus. Acta Diabetol 47, 265–270 (2010). https://doi.org/10.1007/s00592-010-0201-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-010-0201-9