Abstract
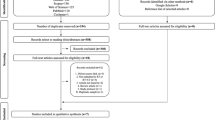
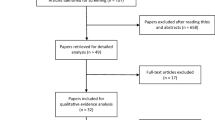
Photobiomodulation therapy (PBMT) has been considered an effective method for preventing and managing certain cancer-related toxicities in head and neck cancer (HNC) patients treated with radiotherapy and chemotherapy. However, the potential effects of PBMT on pain control and analgesia resulting from these toxicities is still controversial. The aim of this systematic review was to compile available evidence of the effects of PMBT on pain control and reduced use of analgesics in HNC patients. We searched three indexed databases: MEDLINE/PubMed, Embase, and Scopus. The databases were reviewed up to and including December 2018. Only human clinical studies in English language were selected. Information was only available for mucositis and radiodermatitis. Fifteen out of 1112 studies met the inclusion criteria (14 for oral mucositis (OM) and 1 for radiodermatitis). From the 14 studies involving the prevention and treatment of OM, 10 had the study subjects compared to a placebo group. Of these 10 studies, all but 1 showed statistically significant difference related to pain control favoring the PBMT group. The study that compared PBMT with other treatment modality showed better results in pain control with PBMT. It appears that PBMT application frequency and potency impact on pain control. The only study involving the prevention and treatment of radiodermatitis was compared to placebo arm and showed statistically significant difference related to pain control favoring the PBMT group. Seven studies compared the need of analgesic medication between PBMT and placebo groups. Of these, five studies showed that the use of analgesic medication was significantly higher in the placebo group. The current evidence supports that PBMT is effective in pain control resulting from OM and radiodermatitis and may also reduce the need for analgesics. The evidence is not yet available of the effects of PBMT in other HNC treatment-related toxicities.


Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2018) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer Oct 23. https://doi.org/10.1002/ijc.31937
Kelly JR, Husain ZA, Burtness B (2016) Treatment de-intensification strategies for head and neck cancer. Eur J Cancer 68:125–133. https://doi.org/10.1016/j.ejca.2016.09.006
Bossi P, Cossu Rocca M, Corvò R, Depenni R, Guardamagna V, Marinangeli F, Miccichè F, Trippa F (2017) The vicious circle of treatment-induced toxicities in locally advanced head and neck cancer and the impact on treatment intensity. Crit Rev Oncol Hematol 116:82–88. https://doi.org/10.1016/j.critrevonc.2017.05.012
Baxter GD, Liu L, Petrich S, Gisselman AS, Chapple C, Anders JJ, Tumilty S (2017) Low level laser therapy (photobiomodulation therapy) for breast cancer-related lymphedema: a systematic review. BMC Cancer 17(1):833. https://doi.org/10.1186/s12885-017-3852-x
Argenta PA, Ballman KV, Geller MA, Carson LF, Ghebre R, Mullany SA, Teoh DGK, Winterhoff BJN, Rivard CL, Erickson BK (2017) The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: a randomized, sham-controlled clinical trial. Gynecol Oncol 144(1):159–166. https://doi.org/10.1016/j.ygyno.2016.11.013
Zhang X, Li H, Li Q, Li Y, Li C, Zhu M et al (2018) Application of red light phototherapy in the treatment of radioactive dermatitis in patients with head and neck cancer. World J Surg Oncol 16(1):222. https://doi.org/10.1186/s12957-018-1522-3
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Guddattu V (2015) Low level laser therapy against radiation induced oral mucositis in elderly head and neck cancer patients-a randomized placebo controlled trial. J Photochem Photobiol B 144:51–56. https://doi.org/10.1016/j.jphotobiol.2015.01.011
Robijns J, Censabella S, Bulens P, Maes A, Mebis J (2017) The use of low-level light therapy in supportive care for patients with breast cancer: review of the literature. Lasers Med Sci 32(1):229–242. https://doi.org/10.1007/s10103-016-2056-y
Strouthos I, Chatzikonstantinou G, Tselis N, Bon D, Karagiannis E, Zoga E, Ferentinos K, Maximenko J, Nikolettou-Fischer V, Zamboglou N (2017) Photobiomodulation therapy for the management of radiation-induced dermatitis: a single-institution experience of adjuvant radiotherapy in breast cancer patients after breast conserving surgery. Strahlenther Onkol 193(6):491–498. https://doi.org/10.1007/s00066-017-1117-x
Weissheimer C, Curra M, Gregianin LJ, Daudt LE, Wagner VP, Martins MAT, Martins MD (2017) New photobiomodulation protocol prevents oral mucositis in hematopoietic stem cell transplantation recipients-a retrospective study. Lasers Med Sci 32(9):2013–2021. https://doi.org/10.1007/s10103-017-2314-7
Cousins MJ, Lynch ME (2011) The Declaration Montreal: access to pain management is a fundamental human right. Pain 152(12):2673–2674. https://doi.org/10.1016/j.pain.2011.09.012
Peterson DE, Boers-Doets CB, Bensadoun RJ, Herrstedt J, Committee EG (2015) Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 26(Suppl 5):139–151. https://doi.org/10.1093/annonc/mdv202
Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M, Dejou J, Tardieu C, Benezery K, Nguyen TD, Laudoyer Y, Dassonville O, Poissonnet G, Vallicioni J, Thyss A, Hamdi M, Chauvel P, Demard F (1999) Low-energy He/Ne laser in the prevention of radiation induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer 7:244–252. https://doi.org/10.1007/s005209900034
Arun Maiya G, Sagar MS, Fernandes D (2006) Effect of low level helium-neon (He-Ne) laser therapy in the prevention & treatment of radiation induced mucositis in head & neck cancer patients. Indian. J Med Res 124(4):399–402
Arora H, Pai KM, Maiya A, Vidyasagar MS, Rajeev A (2008) Efficacy of He-Ne laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105(2):180–6, 186.e1. https://doi.org/10.1016/j.tripleo.2007.07.043
Simões A, Eduardo FP, Luiz AC, Campos L, Sá PH, Cristófaro M et al (2009) Laser phototherapy as topical prophylaxis against head and neck cancer radiotherapy-induced oral mucositis: comparison between low and high/low power lasers. Lasers Surg Med 41(4):264–270. https://doi.org/10.1002/lsm.20758
Lima AG, Antequera R, Peres MP, Snitcosky IM, Federico MH, Villar RC (2010) Efficacy of low-level laser therapy and aluminum hydroxide in patients with chemotherapy and radiotherapy-induced oral mucositis. Braz Dent J 21(3):186–192
Zanin T, Zanin F, Carvalhosa AA, Castro PH, Pacheco MT, Zanin IC et al (2010) Use of 660-nm diode laser in the prevention and treatment of human oral mucositis induced by radiotherapy and chemotherapy. Photomed Laser Surg 28(2):233–237. https://doi.org/10.1089/pho.2008.2242
Carvalho PA, Jaguar GC, Pellizzon AC, Prado JD, Lopes RN, Alves FA (2011) Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: a double-blind randomized study in head and neck cancer patients. Oral Oncol 47(12):1176–1181. https://doi.org/10.1016/j.oraloncology.2011.08.021
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Vadhiraja BM (2012) Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients—a triple blinded randomized controlled trial. Radiother Oncol 104(3):349–354. https://doi.org/10.1016/j.radonc.2012.06.011
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya GA (2012) Low level helium neon laser therapy for chemoradiotherapy induced oral mucositis in oral cancer patients—a randomized controlled trial. Oral Oncol 48(9):893–897. https://doi.org/10.1016/j.oraloncology.2012.03.008
Gouvêa de Lima A, Villar RC, de Castro G Jr, Antequera R, Gil E, Rosalmeida MC et al (2012) Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys 82(1):270–275. https://doi.org/10.1016/j.ijrobp.2010.10.012
Antunes HS, Herchenhorn D, Small IA, Araújo CM, Viégas CM, Cabral E et al (2013) Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother Oncol 109(2):297–302. https://doi.org/10.1016/j.radonc.2013.08.010
Oton-Leite AF, Elias LS, Morais MO, Pinezi JC, Leles CR, Silva MA et al (2013) Effect of low level laser therapy in the reduction of oral complications in patients with cancer of the head and neck submitted to radiotherapy. Spec Care Dentist 33(6):294–300. https://doi.org/10.1111/j.1754-4505.2012.00303.x
Soares RG, Farias LC, da Silva Menezes AS, de Oliveira E, Silva CS, Tabosa ATL et al (2018) Treatment of mucositis with combined 660- and 808-nm-wavelength low-level laser therapy reduced mucositis grade, pain, and use of analgesics: a parallel, single-blind, two-arm controlled study. Lasers Med Sci 33(8):1813–1819. https://doi.org/10.1007/s10103-018-2549-y
Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 18(2):95–99
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23(2):161–166
Tuby H, Maltz L, Oron U (2007) Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med 39(4):373–378
Hou JF, Zhang H, Yuan X, Li J, Wei YJ, SS H (2008) In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med 40(10):726–733
Rocha Junior AM, Vieira BJ, de Andrade LC, Aarestrup FM (2009) Low-level laser therapy increases transforming growth factor-beta2 expression and induces apoptosis of epithelial cells during the tissue repair process. Photomed Laser Surg 27(2):303–307
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16:4. https://doi.org/10.1186/1423-0127-16-4
Hawkins DH, Abrahamse H (2006) The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg Med 38(1):74–83
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 49(1):1–17
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM et al (2014) Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120(10):1453–1461. https://doi.org/10.1002/cncr.28592
Bjordal JM, Bensadoun RJ, Tunèr J, Frigo L, Gjerde K, Lopes-Martins RA (2011) A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer 19(8):1069–1077. https://doi.org/10.1007/s00520-011-1202-0
Fekrazad R, Chiniforush N (2014) Oral mucositis prevention and management by therapeutic laser in head and neck cancers. J Lasers Med Sci 5(1):1–7
Chow RT, Armati PJ (2016) Photobiomodulation: implications for anesthesia and pain relief. Photomed Laser Surg 34(12):599–609. https://doi.org/10.1089/pho.2015.4048
Ferreira DM, Zângaro RA, Villaverde AB, Cury Y, Frigo L, Picolo G, Longo I, Barbosa DG (2005) Analgesic effect of He-Ne (632.8 nm) low-level laser therapy on acute inflammatory pain. Photomed Laser Surg 23(2):177–181
Bensadoun RJ (2018) Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr Opin Oncol 30(4):226–232. https://doi.org/10.1097/CCO.0000000000000452
Oberoi S, Zamperlini-Netto G, Beyene J, Treister NS, Sung L (2014) Effect of prophylactic low level laser therapy on oral mucositis: a systematic review and meta-analysis. PLoS One 9(9):e107418. https://doi.org/10.1371/journal.pone.0107418
Cengiz M, Özyar E, Öztürk D, Akyol F, Atahan IL, Hayran M (1999) Sucralfate in the prevention of radiation-induced oral mucositis. J Clin Gastroenterol 28:40–43
Etiz D, Erkal HS, Serin M, Küçük B, Hepari A, Elhan AH et al (2000) Clinical and histopathological evaluation of sucralfate in prevention of oral mucositis induced by radiation therapy in patients with head and neck malignancies. Oral Oncol 36:116–120
McQuestion M (2011) Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs 27:e1–e17. https://doi.org/10.1016/j.soncn.2011.02.009
Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB et al (2013) Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer 21:2933–2948. https://doi.org/10.1007/s00520-013-1896-2
Franco P, Potenza I, Moretto F, Segantin M, Grosso M, Lombardo A, Taricco D, Vallario P, Filippi AR, Rampino M, Ricardi U (2014) Hypericum perforatum and neem oil for the management of acute skin toxicity in head and neck cancer patients undergoing radiation or chemo-radiation: a single-arm prospective observational study. Radiat Oncol 9:297. https://doi.org/10.1186/s13014-014-0297-0
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N et al (2013) Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 32(1):41–52
Costa MM, Silva SB, Quinto AL, Pasquinelli PF, de Queiroz dos Santos V, de Cassia SG et al (2014) Phototherapy 660 nm for the prevention of radiodermatitis in breast cancer patients receiving radiation therapy: study protocol for a randomized controlled trial. Trials 15:330. https://doi.org/10.1186/1745-6215-15-330
Bensadoun RJ, Nair RG (2015) Low-level laser therapy in the management of mucositis and dermatitis induced by cancer therapy. Photomed Laser Surg 33(10):487–491. https://doi.org/10.1089/pho.2015.4022
Robijns J, Censabella S, Claes S, Pannekoeke L, Bussé L, Colson D, Kaminski I, Bulens P, Maes A, Noé L, Brosens M, Timmermans A, Lambrichts I, Somers V, Mebis J (2018) Prevention of acute radiodermatitis by photobiomodulation: a randomized, placebo-controlled trial in breast cancer patients (TRANSDERMIS trial). Lasers Surg Med 50:763–771. https://doi.org/10.1002/lsm.22804
Buffa FM, Bentzen SM, Daley FM, Dische S, Saunders MI, Richman PI et al (2004) Molecular marker profiles predict locoregional control of head and neck squamous cell carcinoma in a randomize trial of continuous hyperfractionated accelerated radiotherapy. Clin Cancer Res 10:3745–3754
Acknowledgments
The authors would like to gratefully acknowledge the financial support of the São Paulo Research Foundation (FAPESP) processes numbers 2018/02233-6, 2013/18402-8, and 2012/06138-1 as well as the National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Control of the data
The authors have full control of all primary data and agree to allow the journal to review our data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Pauli Paglioni, M., Alves, C.G.B., Fontes, E.K. et al. Is photobiomodulation therapy effective in reducing pain caused by toxicities related to head and neck cancer treatment? A systematic review. Support Care Cancer 27, 4043–4054 (2019). https://doi.org/10.1007/s00520-019-04939-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04939-2