Abstract
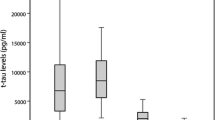
Prion real-time quaking-induced conversion (RT-QuIC) is emerging as the most potent assay for the in vivo diagnosis of Creutzfeldt–Jakob disease (CJD), but its full application, especially as a screening test, is limited by suboptimal substrate availability, reagent costs, and incomplete assay standardization. Therefore, the search for the most informative cerebrospinal fluid (CSF) surrogate biomarker is still of primary importance. We compared the diagnostic accuracy of CSF protein 14-3-3, measured with both western blot (WB) and enzyme-linked immunosorbent assay (ELISA), total (t)-tau and neurofilament light chain protein (NfL) alone or in combination with RT-QuIC in 212 subjects with rapidly progressive dementia in which we reached a highly probable clinical diagnosis at follow-up or a definite neuropathological diagnosis. T-tau performed best as surrogate CSF biomarker for the diagnosis of CJD (91.3% sensitivity and 78.9% specificity). The 14-3-3 ELISA assay demonstrated a slightly higher diagnostic value compared to the WB analysis (76.9% vs. 72.2%), but both methods performed worse than the t-tau assay. NfL was the most sensitive biomarker for all sCJD subtypes (> 95%), including those with low values of t-tau or 14-3-3, but showed the lowest specificity (43.1%). When ELISA-based biomarkers were adopted as screening tests followed by RT-QuIC, t-tau correctly excluded a higher number of non-CJD cases compared to NfL and 14-3-3 ELISA. Our study showed that among the CSF surrogate biomarkers of potential application for the clinical diagnosis of CJD, t-tau performs best either alone or as screening test followed by RT-QuIC as a second-level confirmatory test.

Similar content being viewed by others
References
Baiardi S, Rossi M, Capellari S, Parchi P (2019) Recent advances in the histo-molecular pathology of human prion disease. Brain Pathol 29:278–300
Parchi P, Giese A, Capellari S et al (1999) Classification of sporadic Creutzfeldt–Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46:224–233
Parchi P, Strammiello R, Notari S et al (2009) Incidence and spectrum of sporadic Creutzfeldt–Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol 118:659–671
Parchi P, de Boni L, Saverioni D et al (2012) Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol 124:517–529
Baiardi S, Capellari S, Bartoletti Stella A, Parchi P (2018) Unusual clinical presentations challenging the early clinical diagnosis of Creutzfeldt–Jakob disease. J Alzheimers Dis 64:1051–1065
Zerr I, Kallenberg K, Summers DM et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132:2659–2668
Chohan G, Pennington C, Mackenzie JM et al (2010) The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt–Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 81:1243–1248
Hamlin C, Puoti G, Berri S et al (2012) A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt–Jakob disease. Neurology 79:547–552
Lattanzio F, Abu-Rumeileh S, Franceschini A et al (2017) Prion-specific and surrogate CSF biomarkers in Creutzfeldt–Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 133:559–578
Rudge P, Hyare H, Green A, Collinge J, Mead S (2018) Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry 89:461–466
McGuire LI, Peden AH, Orrú CD et al (2012) Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt–Jakob disease. Ann Neurol 72:278–285
Groveman BR, Orrú CD, Hughson AG et al (2016) Extended and direct evaluation of RT-QuIC assays for Creutzfeldt–Jakob disease diagnosis. Ann Clin Transl Neurol 4:139–144
Foutz A, Appleby BS, Hamlin C et al (2017) Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol 81:79–92
Franceschini A, Baiardi S, Hughson AG et al (2017) High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci Rep 7:10655
Schmitz M, Ebert E, Stoeck K et al (2016) Validation of 14-3-3 protein as a marker in sporadic Creutzfeldt–Jakob disease diagnostic. Mol Neurobiol 53:2189–2199
Leitão MJ, Baldeiras I, Almeida MR et al (2016) Sporadic Creutzfeldt–Jakob disease diagnostic accuracy is improved by a new CSF ELISA 14-3-3γ assay. Neuroscience 322:398–407
Steinacker P, Blennow K, Halbgebauer S et al (2016) Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt–Jakob disease. Sci Rep 6:38737
Abu-Rumeileh S, Capellari S, Stanzani-Maserati M et al (2018) The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther 10:3
Zerr I, Schmitz M, Karch A et al (2018) Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimers Dement 14:751–763
Kanata E, Golanska E, Villar-Piqué A et al (2019) Cerebrospinal fluid neurofilament light in suspected sporadic Creutzfeldt–Jakob disease. J Clin Neurosci 60:124–127
Grau-Rivera O, Gelpi E, Nos C et al (2015) Clinicopathological correlations and concomitant pathologies in rapidly progressive dementia: a brain bank series. Neurodegener Dis 15:350–360
Geschwind MD, Murray K (2018) Differential diagnosis with other rapid progressive dementias in human prion diseases. Handb Clin Neurol 153:371–397
Zerr I, Hermann P (2018) Diagnostic challenges in rapidly progressive dementia. Expert Rev Neurother 18:761–772
Jansen C, Parchi P, Capellari S et al (2012) Human prion diseases in the Netherlands (1998–2009): clinical, genetic and molecular aspects. PLoS ONE 7:e36333
ECDC (2017) https://www.cjd.ed.ac.uk/sites/default/files/criteria_0.pdf. Accessed 10 July 2019
McGuire LI, Poleggi A, Poggiolini I et al (2016) Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic Creutzfeldt–Jakob disease: an international study. Ann Neurol 80:160–165
Otto M, Wiltfang J, Cepek L et al (2002) Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt–Jakob disease. Neurology 58:192–197
Skillbäck T, Rosén C, Asztely F et al (2014) Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt–Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol 71:476–483
Gaetani L, Blennow K, Calabresi P et al (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90:870–881
Kovacs GG, Andreasson U, Liman V et al (2017) Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol 24:1326–e77
Acknowledgements
We sincerely thank Dr. Byron Caughey for providing the substrate for IQ-CSF RT-QuIC. This work was supported by the Italian Ministry of Health (“Ricerca Corrente”).
Author information
Authors and Affiliations
Contributions
Conceptualization: SA-R and PP; methodology, formal analysis and investigation: all authors; writing—original draft preparation: SA-R and PP; writing—review and editing: PP based on the critical revision of all authors—supervision: PP.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines and approved by the “Area Vasta Emilia Centro” ethics committee (CE-AVEC: 18025, 113/2018/OSS/AUSLBO). Informed consent was given by study participants or the next of kin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abu-Rumeileh, S., Baiardi, S., Polischi, B. et al. Diagnostic value of surrogate CSF biomarkers for Creutzfeldt–Jakob disease in the era of RT-QuIC. J Neurol 266, 3136–3143 (2019). https://doi.org/10.1007/s00415-019-09537-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09537-0