Abstract
Aim
The purpose of this work was to prove the validity of the mean lung dose (MLD), widely used in clinical practice to estimate the lung toxicity of a treatment plan, by reevaluating experimental data from mini pigs.
Materials and methods
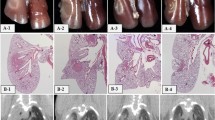
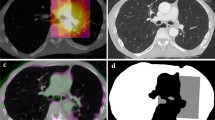
A total of 43 mini pigs were irradiated in one of four dose groups (25, 29, 33, and 37 Gy). Two regimens were applied: homogeneous irradiation of the right lung or partial irradiation of both lungs—including parts with lower dose—but with similar mean lung doses. The animals were treated with five fractions with a linear accelerator applying a CT-based treatment plan. The clinical lung reaction (breathing frequency) and morphological changes in CT scans were examined frequently during the 48 weeks after irradiation.
Results
A clear dose–effect relationship was found for both regimens of the trial. However, a straightforward relationship between the MLD and the relative number of responders with respect to different grades of increased breathing frequency for both regimens was not found. A morphologically based parameter NTCPlung was found to be more suitable for this purpose. The dependence of this parameter on the MLD is markedly different for the two regimens.
Conclusion
In clinical practice, the MLD can be used to predict lung toxicity of a treatment plan, except for dose values that could lead to severe side effects. In the latter mentioned case, limitations to the predictive value of the MLD are possible. Such severe developments of a radiation-induced pneumopathy are better predicted by the NTCPlung formalism. The predictive advantage of this parameter compared to the MLD seems to remain in the evaluation and comparison of widely differing dose distributions, like in the investigated trial.
Zusammenfassung
Zielsetzung
Es soll unter Reevaluation von Tierversuchsdaten am Minischwein geprüft werden, ob die in der klinischen Praxis zur Beurteilung der Lungentoxizität eines Bestrahlungsregims regelhaft verwendete mittlere Lungendosis (MLD) eine zuverlässige Voraussage der Lungenbelastung erlaubt.
Material und Methoden
Insgesamt 43 Minischweine wurden in vier Dosisgruppen (25, 29, 33 und 37 Gy) entweder mit einer homogenen Bestrahlung der gesamten rechten Lunge oder mit einer Teillungenbestrahlung (jeweils Anteile beider Lungen auch mit niedrigerer Dosis) mit vergleichbarer MLD nach CT-gestützter Bestrahlungsplanung am Linearbeschleuniger in fünf Fraktionen bestrahlt. Die Tiere wurden 48 Wochen nachbeobachtet und in regelmäßigen Abständen sowohl hinsichtlich ihrer klinischen Lungenreaktion (Atemfrequenzerhöhung) als auch der im CT nachweisbaren bildmorphologischen Veränderungen kontrolliert.
Ergebnisse
Es besteht eine klare Dosis-Effekt-Beziehung in beiden Versuchsarmen. Allerdings zeigte sich kein eindeutiger Zusammenhang zwischen der MLD und dem relativen Response hinsichtlich verschiedener Grade der Atemfrequenzerhöhung für beide Versuchsarme. Hierfür scheint ein morphologisch basierter Parameter NTCPlung besser geeignet zu sein. Der Verlauf dieses Parameters in Abhängigkeit der MLD unterscheidet sich deutlich für beide Versuchsarme.
Schlussfolgerung
Die MLD kann in der klinischen Praxis für die Vorhersage der Lungentoxizität eines Bestrahlungsregims genutzt werden, sofern in Abhängigkeit von der zu applizierenden Dosis nicht mit einer schwereren Nebenwirkung gerechnet wird. Sind jedoch schwerere Lungenreaktionen zu erwarten, so sind Einschränkungen hinsichtlich der Vorhersage der Lungentoxizität mittels MLD möglich. Schwere Radiopneumonitisverläufe werden besser durch einen NTCPlung-Wert beschrieben. Dieser Vorteil scheint auch bei der Bewertung und dem Vergleich stark differierender Dosisverteilungen, wie im vorliegenden Versuch, erhalten zu bleiben.




Similar content being viewed by others
References
Eldh T, Heinzelmann F, Velakan A, Budach W, Belka C, Jendrossek V (2012) Radiation-induced changes in breathing frequency and lung histology of C57BL/6J mice are time- and dose-dependent. Strahlenther Onkol 188:274–281
Ghobadi G, van der Veen S, Bartelds B, de Boer R A, Dickinson M G, de Jong J R, Faber H, Niemantsverdriet M, Brandenburg S, Berger R M F, Langendijk J A, Coppes R P, van Luijk P (2012) Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 84:639–646
Guckenberger M, Kavanagh A, Partridge M (2012) Combining advanced radiotherapy technologies to maximize safety and tumor control probability in stage III non-small cell lung cancer. Strahlenther Onkol 188:894–900
Guckenberger M, Klement R J, Kestin L, Hope AJ, Belderbos J, Werner-Wasik M, Yan D, Sonke JJ, Bissonnette JP, Xiao Y, Grills IS (2013) Lack of a dose-effect relationship for pulmonary function changes after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 85:1074–1081
Herrmann Th, Voigtmann L, Knorr A, Lorenz J, Johannsen U (1986) Dose-time relationship of the radiogenic lung reaction in pigs. Radiother Oncol 5:127–135
Herrmann Th, Knorr A (1995) Die radiogene Lungenreaktion, Pathogenese-Prävention-Therapie. Strahlenther Onkol 171:490–498
Herrmann Th, Baumann M, Voigtmann L, Knorr A (1997) Effect of irradiated volume on lung damage in pigs. Radiother Oncol 44:35–40
Kellerer A M, Rossi HH (1972) The theory of dual radiation action. Curr Topic Radiat Res Q 8:85–158
Liao ZX, Travis EL, Tucker SL (1995) Damage and morbidity from pneumonitis after irradiation of partial volumes of mouse lung. Int J Radiat Oncol Biol Phys 32: 1359–1370
Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, Albain K, Sause WT, Curran WJ (2012) Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys 82:425–434
Marchand V, Zefkili S, Desrousseaux J, Simon l, Dauphinot C, Giraud P (2012) Dosimetric comparison of free-breathing and deep inspiration breath-hold radiotherapy for lung cancer. Strahlenther Onkol 188:582–591
Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK (2010) Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 76:S70–S76
McLaughlin RF, Tyler WS, Canada RO (1961) A study of the subgross pulmonary anatomy in various mammals. Am J Anat 108:149–165
Moustafa HF, Hopewell JW (1977) Measurements of lung function in the pig after local X-irradiation. Research and Radiother. IAEA-SM212/45 Wien 1:75–84
Novakova-Jiresova A, van Luijk P, van Goor H, Kampinga HH, Coppse RP (2005) Pulmonary radiation injury: identification of risk factors associated with regional hypersensitivity. Cancer Res 65:3568–3576
Palma DA, Sehan S, Haasbeek CJA, Verbakel WFAR, Vincent A (2011). Radiological and clinical pneumonitis after stereotactic lung radiotherapy: a matched analysis of three-dimensional conformal and volumetric-modulated arc therapy techniques. Int J Radiat Oncol Biol Phys 80:506–513
Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, de Petris L, Stitt L, Rodrigues G (2013) Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 85:444–450
Roeder F, Friedrich J, Timke C, Kappes J, Huber P, Krempien R, Debus J, Bischof M (2010) Correlation of patient-related factors and dose-volume histogram parameters with the onset of radiation pneumonitis in patients with small cell lung cancer. Strahlenther Onkol 186:149–156
Schallenkamp JM, Miller RC, Brinkmann D (2007) et al Incidence of radiation pneumonitis after thoracic irradiation: dose-volume correlates. Int J Radiat Oncol Biol Phys 67:410–6
Semenenko VA, Molthen RC, Li C, Morrov NV, Li R, Ghosh SN, Medhora MM, Li XA (2008) Irradiation of Varying volumes of rat lung to same mean lung dose: a little to a lot or a lot to a little? Int J Radiat Oncol Biol Phys 71:838–847
Seppenwoolde Y, De Jaeger K, Boersma LJ, Belderbos JSA, Lebesque JV (2004) Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 60:748–758
Tucker SL, Hekun J, Xiong W, Wang S, Martel M K, Komaki R, Liu H, Mohan R, Chen Y, Cox JD, Liao Z (2010) Impact of toxicity grade and scoring systems on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non-small lung cancer. Int J Radiat Oncol Biol Phys 77:692–698
Tucker SL, Mohan R, Liengswangwong R, Martel MK, Zhongxing L (2013) Predicting pneumonitis risk: a dosimetric alternative to mean lung dose. Int J Radiat Oncol Biol Phys 85:522–527
Van Elmpt W, de Ruysscher D, van der Salm A, Lakeman A, van der Stoep J, Emans D, Damen E, Öllers M, Sonke JJ, Belderbos J (2012) The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol 104:67–71
Vogelius IS, Westerly DC, Cannon GM, Mackie TR, Mehta MM, Sugie C, Bentzen SM (2011) Intensity-modulated radiotherapy might increase pneumonitis risk relative to three-dimensional conformal radiotherapy in patients receiving combined chemotherapy and radiotherapy: a modelling study of dose dumping. Int J Radiat Oncol Biol Phys 80:893–899
Willner J, Jost A, Baier K (2003) et al A little to a lot or a lot to a little? An analysis of pneumonitis risk from dose-volume histogram parameters of the lung in patients with lung cancer treated with 3-D conformal radiotherapy. Strahlenther Onkol 179:548–556
Wurstbauer K, Weise H, Deutschmann H, Kopp P, Merz F, Studnicka M, Nairz O, Sedlmayer F (2010) Non-small cell lung cancer in stages I-IIIB. Strahlenther Onkol 186:551–557
Acknowledgements
Funded by the Federal Ministry of Education and Research as part of the “Lung Fibrosis” Clinical Research Association at the medical faculty of Dresden University of Technology (Funding code 01 ZZ 9604).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Th. Herrmann, P. Geyer, and S. Appold state that there are no conflicts of interest.
All institutional and national guidelines for the care and use of test animals were followed. The permission for the animal experiment was given by the responsible authority (Regierungspräsidium Dresden, No. 75–9168.11-1-2000-9 and 75-9168.11-1.14/9)
Additional information
The authors are grateful to Dr. med. vet. R. Kumpf (†) for his support in carrying out the animal testing.
Appendices
Appendix
A: Modeling of the dependence of NTCPlung on the MLD for the investigated trial
Accordingly to the beam arrangements of chapter 2 the MLD for a prescribed dose value D for the regimen A is in principle given by
with Vr: volume of the right lung,
Vlung: volume of the whole lung, Vlung = Vr + Vl with Vl: volume of the left lung.
For the regimen B, the MLD can be expressed by the equation
with 1/n: irradiated part of the left lung.
For an animal of regimen A, equation (1) leads to the relation
For regimen B, the equation (1) is simplified to
Although the condition of equal values of the MLD for both regimens was not completely achievable, like stated in the Materials and methods section, the validity of this condition will be used for the following simplified modeling. It should be noted that this modeling is limited to the investigated doses and volumes in the regimens. The condition of equal values of the MLD(D) for equal values of the prescribed dose D for both regimens A and B leads to the following equation (by equating equations (A1) and (A2)):
Thus, from Eq. (A5) one gets for the value n the expression
Introducing this expression for n into Eq. (A4) simplifies the formula of NTCPlung, B as follows:
Now, one obtains a relation between NTCPlung and the MLD(D) by applying the condition of equal values of the MLD(D) for both regimens. So Eq. (A1) leads to
and with this expression substituting Vr/ Vlung in equations (A3) and (A7), the following equations for NTCPlung are obtained:
For the same value of MLD(D), the values of NTCPlung for both regimens will only become equal under the condition that
For a more quantitative modeling, the dependence of W(D) accordingly to Table 3 is approximated by a polynom of grade 3 (for 14Gy < D < 38Gy)
Using this expression for W(D) in equations (A9) and (A10) and the matching pairs of values of D and MLDmean(D) from Table 1 leads to the results of Fig. 5a. These results are quite similar to the behavior of the individual relations of NTCPlung dependent on MLD for both regimens shown in Fig. 4, thereby supporting the clearly different but approximately parallel directions of the graphs for both regimens in this figure. To obtain an estimation of the behavior of the NTCPlung beyond the investigated dose range, the D–MLDmean pairs of Table 1 are extended towards lower and higher doses. D is decreased to 10 Gy and increased to 45 Gy. The matching value of MLDmean is estimated as 0.58 * D, the average ratio one gets from Table 1. According to Eq. (A1) this ratio describes the mean value of the ratio of Vr/ Vlung of all animals in the trial. Repeating the NTCPlung calculations with this extended dose range leads to the results presented in Fig. 5b. Here one detects the fundamental sigmoidal character of the NTCPlung graphs with the linear part in the investigated dose range of the trial. The graph for regimen A will reach a maximum value of 0.58 if the alone irradiated right lung has a probability W equal to 1 (maximum complication), which refers to Eq. (A3) with the above mentioned ratio of Vr/ Vlung. The graph for regimen B will further increase with MLDmean, but for this range the approximation of Eq. (A12) is not valid. Even so, one will expect that the NTCPlung graph of regimen B in Fig. 5b will reach and intersect the NTCPlung graph of regimen A at its plateau value of 0.58. Thus, Fig. 5b indicates that—beyond the part of the linear and nearly parallel behavior with the strong separation of both graphs—dose regions exist where both graphs are approaching: for MLDmean < 10 Gy and MLDmean of about 30 Gy. These are the dose regions where condition (A11) is fulfilled, here the NTCPlung is either near to zero or very high.
a Modeling: Dependence of NTCPlung on MLD for both regimens of the trial and for the applied range of the MLD (characterized by a linear behavior). The functions of the linear regressions and the RSQ function (R 2, Excel) are also given. b Modeling: Dependence of NTCPlung on MLD for both regimens of the trial and for an extended range of the MLD
B: Extension of this model to a clinical fractionation pattern
Conventional curative irradiation of a lung tumor could be done by applying 33 fractions with 2 Gy per fraction to a dose reference point in the target. The overall value of the MLD in this case will commonly be limited to a maximum value of about 20 Gy what is equal to a MLD per fraction of about 0.6 Gy. To compare the fractionation pattern of the investigated trial with this clinical case the prescribed doses D of the trial are transformed to overall doses D0.6Gy(D) that will result in the same response to the lung like the dose D, given in five fractions, but with a dose per fraction of 0.6 Gy. This transformation is done using the well-established linear-quadratic (LQ) model [8] with a value of α/ β = 3Gy. Table 4 shows this values D0.6Gy(D), further the value of D/2 and additionally the probabilities W(D)/2 and W(D/2) according to condition (A11), presenting very different values for both probabilities. To characterize the mentioned curative lung tumor irradiation, the value of D0.6Gy(D) = 19 Gy is used in Table 4. This clinical dose constraint corresponds in the used model to a prescribed dose D = 12.5 Gy, given in five fractions. This dose value is much lower than the applied doses D in the trial. For this pair of doses D0,6Gy(D) = 19 Gy and D = 12.5 Gy the probability values W of both regimens of the trial in Table 4 are equal to zero, thereby indicating that there is no difference between the NTCPlung values for the two regimens but also showing that for this dose no complication will expected in the used score (see Table 3).
Rights and permissions
About this article
Cite this article
Herrmann, T., Geyer, P. & Appold, S. The mean lung dose (MLD). Strahlenther Onkol 191, 557–565 (2015). https://doi.org/10.1007/s00066-015-0833-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0833-3