Abstract
Purpose
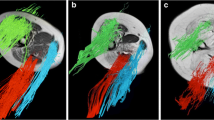
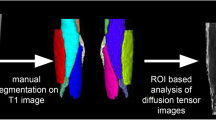
To compare the influence of two different regions of interest (ROIs) on diffusion tensor metrics in dystrophic thigh muscles using a custom-made (whole muscle) ROI including and a selective ROI excluding areas of fatty replacement.
Methods
Diffusion tensor imaging (DTI) and chemical-shift-encoded water-fat magnetic resonance imaging (MRI) of the thigh was conducted on a 3-Tesla system in 15 cases with muscular dystrophy and controls. The ROIs were chosen according to patterns of fatty replacement on co-registered axial DTI and gradient echo sequence (GRE) images. Fractional anisotropy (FA), apparent diffusion coefficient (ADC), fiber track length (FTL), and muscle fat fractions (MFF) were compared between both ROI segmentations. These comparisons, muscle-specific correlation coefficients, and the influence of ROI localization on tensor metrics were derived based on linear mixed effects regression models.
Results
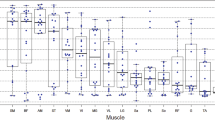
In the cases a high correlation was observed for ADC and FA with MFF using a custom ROI. The correlation was weaker but still significant with a selective ROI method. Using the custom ROI, FTL correlated significantly with MFF in 3 out of 4 muscles (r ≤ −0.51). A correlation was not found for the selective ROI method. Interaction analysis revealed that the association of ADC and FA with MFF was not significantly influenced by the ROI localization. For FTL the ROI localization significantly reduced the negative association with MFF.
Conclusion
The DTI metrics and FTL of custom ROI segmentation are significantly influenced by MFF. Contrary to ADC and FA, the effect of MFF on FTL is significantly reduced when applying selective ROI segmentation, which could therefore be a better option for MR tractography.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BF:
-
Biceps femoris muscle
- DTI:
-
Diffusion tensor imaging
- EPI:
-
Echo planar imaging
- FA:
-
Fractional anisotropy
- FTL:
-
Fiber track length
- G:
-
Gracilis muscle
- GRE:
-
Gradient echo sequence
- MFF:
-
Muscle fat fraction
- NSA:
-
Number of signal averages
- RF:
-
Rectus femoris muscle
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- ST:
-
Semitendinosus muscle
References
Ward SR, Winters TM, Blemker SS. The architectural design of the gluteal muscle group: implications for movement and rehabilitation. J Orthop Sports Phys Ther. 2010;40:95–102.
Bodine SC, Roy RR, Meadows DA, Zernicke RF, Sacks RD, Fournier M, Edgerton VR. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982;48:192–201.
Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–90.
Oudeman J, Nederveen AJ, Strijkers GJ, Maas M, Luijten PR, Froeling M. Techniques and applications of skeletal muscle diffusion tensor imaging: a review. J Magn Reson Imaging. 2016;43:773–88.
Ahmad CS, Redler LH, Ciccotti MG, Maffulli N, Longo UG, Bradley J. Evaluation and management of hamstring injuries. Am J Sports Med. 2013;41:2933–47.
Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed. 2002;15:456–67.
Galbán CJ, Maderwald S, Stock F, Ladd ME. Age-related changes in skeletal muscle as detected by diffusion tensor magnetic resonance imaging. J Gerontol A Biol Sci Med Sci. 2007;62:453–8.
Galbán CJ, Maderwald S, Uffmann K, Ladd ME. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed. 2005;18:489–98.
Ponrartana S, Ramos-Platt L, Wren TA, Hu HH, Perkins TG, Chia JM, Gilsanz V. Effectiveness of diffusion tensor imaging in assessing disease severity in Duchenne muscular dystrophy: preliminary study. Pediatr Radiol. 2015;45:582–9.
Saotome T, Sekino M, Eto F, Ueno S. Evaluation of diffusional anisotropy and microscopic structure in skeletal muscles using magnetic resonance. Magn Reson Imaging. 2006;24:19–25.
Sinha S, Sinha U, Edgerton VR. In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging. 2006;24:182–90.
Damon BM, Ding Z, Anderson AW, Freyer AS, Gore JC. Validation of diffusion tensor MRI-based muscle fiber tracking. Magn Reson Med. 2002;48:97–104.
Mori S, van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15:468–80.
Froeling M, Nederveen AJ, Nicolay K, Strijkers GJ. DTI of human skeletal muscle: the effects of diffusion encoding parameters, signal-to-noise ratio and T2 on tensor indices and fiber tracts. NMR Biomed. 2013;26:1339–52.
Hooijmans MT, Damon BM, Froeling M, Versluis MJ, Burakiewicz J, Verschuuren JJ, Niks EH, Webb AG, Kan HE. Evaluation of skeletal muscle DTI in patients with duchenne muscular dystrophy. NMR Biomed. 2015;28:1589–97.
Williams SE, Heemskerk AM, Welch EB, Li K, Damon BM, Park JH. Quantitative effects of inclusion of fat on muscle diffusion tensor MRI measurements. J Magn Reson Imaging. 2013;38:1292–7.
Gaeta M, Scribano E, Mileto A, Mazziotti S, Rodolico C, Toscano A, Settineri N, Ascenti G, Blandino A. Muscle fat fraction in neuromuscular disorders: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification—a feasibility study. Radiology. 2011;259:487–94.
Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–94.
Hooijmans MT, Niks EH, Burakiewicz J, Anastasopoulos C, van den Berg SI, van Zwet E, Webb AG, Verschuuren JJGM, Kan HE. Non-uniform muscle fat replacement along the proximodistal axis in Duchenne muscular dystrophy. Neuromuscul Disord. 2017;27:458–64.
Díaz-Manera J, Llauger J, Gallardo E, Illa I. Muscle MRI in muscular dystrophies. Acta Myol. 2015;34:95–108.
Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011;65:96–107.
Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9.
Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23.
Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28:543–58.
R Development Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016.
Gamer M, Lemon J, Fellows I, Singh P. irr: various coefficients of interrater reliability and agreement. R package version 0.84. 16.07.2012 http://CRAN.R-project.org/package=irr. Accessed: 16.07.2012
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Chan WP, Liu GC. MR imaging of primary skeletal muscle diseases in children. AJR Am J Roentgenol. 2002;179:989–97.
Janssen BH, Voet NB, Nabuurs CI, Kan HE, de Rooy JW, Geurts AC, Padberg GW, van Engelen BG, Heerschap A. Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One. 2014;9:e85416.
Regula JU, Jestaedt L, Jende F, Bartsch A, Meinck HM, Weber MA. Clinical muscle testing compared with whole-body magnetic resonance imaging in facio-scapulo-humeral muscular dystrophy. Clin Neuroradiol. 2016;26:445–55.
Li GD, Liang YY, Xu P, Ling J, Chen YM. Diffusion-tensor imaging of thigh muscles in Duchenne muscular dystrophy: correlation of apparent diffusion coefficient and fractional anisotropy values with fatty infiltration. AJR Am J Roentgenol. 2016;206:867–70.
Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906.
Urtasun M, Sáenz A, Roudaut C, Poza JJ, Urtizberea JA, Cobo AM, Richard I, García Bragado F, Leturcq F, Kaplan JC, Martí Massó JF, Beckmann JS, López de Munain A. Limb-girdle muscular dystrophy in Guipuzcoa (Basque country, Spain). Brain. 1998;121(Pt 9):1735–47.
Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24:482–91.
Acknowledgements
We kindly thank Dr. Jan Sedlacik (Department of Neuroradiology, University Medical Center Hamburg-Eppendorf) for the critical review of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Keller, Z.J. Wang, A. Aigner, A.C. Kim, A. Golsari, G. Adam and J. Yamamura declare that they have no competing interests. H. Kooijman is an employee of Philips Healthcare.
Caption Electronic Supplementary Material
Supplement Table 1.
Pearson correlation coefficientsa of ADC, FA, FTL, MFF, and demographics in cases and controls (aBased on mixed effects models with random intercepts for person)
Supplement Table 2:
Standard deviation of three technical replicate regions-of-interest used for the custom and selective ROI method in cases
Supplement Table 3.
Correlation analysis of custom and selective ROI DTI metrics and FTL
Rights and permissions
About this article
Cite this article
Keller, S., Wang, Z.J., Aigner, A. et al. Diffusion Tensor Imaging of Dystrophic Skeletal Muscle. Clin Neuroradiol 29, 231–242 (2019). https://doi.org/10.1007/s00062-018-0667-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0667-3