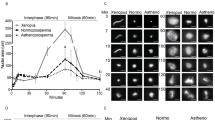
We studied the effect of extracellular vesicles of the follicular fluid on morphofunctional characteristics of human spermatozoa using CASA (computer-assisted sperm analysis) analytical system. The vesicles were obtained by sequential centrifugation at different rotational speeds and frozen at -80°C in the Sydney IVF Gamete Buffer medium. The sperm fraction was isolated from the seminal fluid of 21 patients aged 27-36 years by differential centrifugation in a density gradient. The precipitate was suspended in Sydney IVF Gamete Buffer to a concentration of 106/ml and incubated with vesicles (1:2) at 37°C in a CO2 incubator for 30 min and 1 h. Sperm fraction incubated without vesicles served as the control. After incubation, some sperm samples were centrifuged at 700g for 5 min and fixed in 2.5% glutaraldehyde in 0.1 M buffer for transmission electron microscopy. After 30-min and 1-h incubation, the progressive and total sperm motility improved, the curvilinear and linear velocity of spermatozoa did not change significantly. Incubation with vesicles significantly changed the trajectory of sperm movement, which can attest to an increase in their hyperactivation and, probably, fertilizing capacity. Analysis of the effect of extracellular vesicles of follicular fluid on sperm motility will help to improve the effectiveness of assisted reproductive technology programs with male infertility factor by improving sperm characteristics in patients with asthenozoospermia and increasing the fertilizing ability of the sperm.
Similar content being viewed by others
References
Kraevaya EE, Silachev DN, Beznoshchenko OS, Goryunov KV, Shevtsova JA, Khutornenko AA, Makarova NP, Krechetova LV, Ivanets TY, Poletaev AV, Kalinina EA, Dolgushina NV, Sukhikh GT. Effect of extracellular vesicles of follicular fluid on ovarian coagulation hemostasis. Problemy Reproduktsii. 2020;26(2):18-26. doi: https://doi.org/10.17116/repro20202602118. Russian.
Al-Dossary AA, Bathala P, Caplan JL, Martin-DeLeon PA. Oviductosome-sperm membrane interaction in cargo delivery: Detection of fusion and underlying molecular players using three-dimensional super-resolution structured illumination microscopy (SR-SIM). J. Biol. Chem. 2015;290(29):17710- 17723. doi: https://doi.org/10.1074/jbc.M114.633156
Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One. 2013;8(11):e80181. doi: https://doi.org/10.1371/journal.pone.0080181
Almiñana C, Corbin E, Tsikis G, Alcântara-Neto A.S, Labas V, Reynaud K, Galio L, Uzbekov R, Garanina A. S, Druart X, Mermillod P. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction. 2017;154(3):153-168. doi: https://doi.org/10.1530/REP-17-0054
Ambekar AS, Nirujogi RS, Srikanth SM, Chavan S, Kelkar DS, Hinduja I, Zaveri K, Prasad TS, Harsha HC, Pandey A, Mukherjee S. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J. Proteomics. 2013;87:68-77. doi: https://doi.org/10.1016/j.jprot.2013.05.017
Candenas L, Chianese R. Exosome composition and seminal plasma proteome: a promising source of biomarkers of male infertility. Int. J. Mol. Sci. 2020;21(19):7022. doi: https://doi.org/10.3390/ijms21197022
Chen MS, Tung KS, Coonrod SA, Takahashi Y, Bigler D, Chang A, Yamashita Y, Kincade PW, Herr JC, White JM. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc. Natl Acad. Sci. USA. 1999;96(21):11830-11835. doi: https://doi.org/10.1073/pnas.96.21.11830
da Silveira JC, Carnevale EM, Winger QA, Bouma GJ. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod. Biol. Endocrinol. 2014;12:44. doi: https://doi.org/10.1186/1477-7827-12-44
da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012;86(3):71. doi: https://doi.org/10.1095/biolreprod.111.093252
Dun MD, Smith ND, Baker MA, Lin M, Aitken RJ, Nixon B. The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. J. Biol. Chem. 2011;286(42):36875-36887. doi: https://doi.org/10.1074/jbc.M110.188888
Fabbri R, Porcu E, Lenzi A, Gandini L, Marsella T, Flamigni C. Follicular fluid and human granulosa cell cultures: influence on sperm kinetic parameters, hyperactivation, and acrosome reaction. Fertil. Steril. 1998;69(1):112-117. doi: https://doi.org/10.1016/s0015-0282(97)00421-4
Ferraz MAMM, Carothers A, Dahal R, Noonan MJ, Songsasen N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019;9(1):9484. doi: https://doi.org/10.1038/s41598-019-45857-x
Getpook C, Wirotkarun S. Sperm motility stimulation and preservation with various concentrations of follicular fluid. J. Assist. Reprod. Genet. 2007;24(9):425-448. doi: https://doi.org/10.1007/s10815-007-9145-6
Hernández Alvarado SR, Guzmán-Grenfell AM, Hicks Gómez JJ. Communication of gametes at distance. Chemotaxis and chemokinesis in spermatozoa in mammals. Ginecol. Obstet. Mex. 1995;63:323-327.
Jeon BG, Moon JS, Kim KC, Lee HJ, Choe SY, Rho GJ. Follicular fluid enhances sperm attraction and its motility in human. J. Assist. Reprod. Genet. 2001;18(8):407-412. doi: https://doi.org/10.1023/a:1016674302652
Kenigsberg S, Wyse BA, Librach CL, da Silveira JC. Protocol for exosome isolation from small volume of ovarian follicular fluid: evaluation of ultracentrifugation and commercial kits. Methods Mol. Biol. 2017;1660:321-341. doi: https://doi.org/10.1007/978-1-4939-7253-1_26
Krausz C, Gervasi G, Forti G, Baldi E. Effect of plateletactivating factor on motility and acrosome reaction of human spermatozoa. Hum. Reprod. 1994;9(3):471-476. doi: https://doi.org/10.1093/oxfordjournals.humrep.a138529
Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update. 2016;22(2):182-193. doi: https://doi.org/10.1093/humupd/dmv055
Murdica V, Giacomini E, Alteri A, Bartolacci A, Cermisoni GC, Zarovni N, Papaleo E, Montorsi F, Salonia A, Viganò P, Vago R. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019;111(5):897-908.e2. doi: https://doi.org/10.1016/j.fertnstert.2019.01.030
Navakanitworakul R, Hung WT, Gunewardena S, Davis JS, Chotigeat W, Christenson LK. Characterization and small RNA content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci. Rep. 2016;6:25486. doi: https://doi.org/10.1038/srep25486
Ralt D, Goldenberg M, Fetterolf P, Thompson D, Dor J, Mashiach S, Garbers DL, Eisenbach M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc. Natl Acad. Sci. USA. 1991;88(7):2840-2844. doi: https://doi.org/10.1073/pnas.88.7.2840
Ralt D, Manor M, Cohen-Dayag A, Tur-Kaspa I, Ben-Shlomo I, Makler A, Yuli I, Dor J, Blumberg S, Mashiach S. et al. Chemotaxis and chemokinesis of human spermatozoa to follicular factors. Biol. Reprod. 1994;50(4):774-785. doi: https://doi.org/10.1095/biolreprod50.4.774
Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Xing Q, Jin L, He L, Wu L, Wang L. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013;98(7):3068-3079. doi: https://doi.org/10.1210/jc.2013-1715.
Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, Neyses L. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 2004;279(27):28 220-28 226. doi: https://doi.org/10.1074/jbc.M312599200
Shen X, Liu X, Zhu P, Zhang Y, Wang J, Wang Y, Wang W, Liu J, Li N, Liu F. Proteomic analysis of human follicular fluid associated with successful in vitro fertilization. Reprod. Biol. Endocrinol. 2017;15(1):58. doi: https://doi.org/10.1186/s12958-017-0277-y
Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018;39(3):292-332. doi: https://doi.org/10.1210/er.2017-00229
Visconti PE. Sperm bioenergetics in a nutshell. Biol. Reprod. 2012;87(3):72. doi: https://doi.org/10.1095/biolreprod.112.104109
Williams M, Hill CJ, Scudamore I, Dunphy B, Cooke ID, Barratt CL. Sperm numbers and distribution within the human fallopian tube around ovulation. Hum. Reprod. 1993;8(12):2019- 2026. doi: https://doi.org/10.1093/oxfordjournals.humrep.a137975
Yao Y, Ho P, Yeung WS. Effects of human follicular fluid on the capacitation and motility of human spermatozoa. Fertil. Steril. 2000;73(4):680-686. doi: https://doi.org/10.1016/s0015-0282(99)00637-8
Zhang X, Song D, Kang H, Zhou W, Chen H, Zeng X. Seminal plasma exosomes evoke calcium signals via the CatSper channel to regulate human sperm function. BioRxiv. 2020. doi: https://doi.org/10.1101/2020.05.21.094433
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 3, pp. 176-185, September, 2021
Rights and permissions
About this article
Cite this article
Sysoeva, A.P., Makarova, N.P., Silachev, D.N. et al. Influence of Extracellular Vesicles of the Follicular Fluid on Morphofunctional Characteristics of Human Sperm. Bull Exp Biol Med 172, 254–262 (2021). https://doi.org/10.1007/s10517-021-05372-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-021-05372-4