Root-Associated Mycobiome Differentiate between Habitats Supporting Production of Different Truffle Species in Serbian Riparian Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling of Roots and Soil
2.3. Soil Analyses
2.4. DNA Isolation and Illumina Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Characterization
3.2. Overview of Sequencing Dataset
3.3. Alpha Diversity and Community Composition of Root-Associated Mycobiome in Different Truffle-Producing Riparian Forest Types
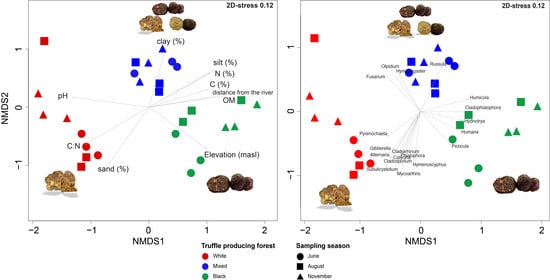
3.4. Beta Diversity and Correlation between Root-Associated Mycobiomes and Environmental Physicochemical Variables
3.5. Core Mycobiome Across Forests Types and Sampling Seasons
3.6. Distribution of Fungal Trophic Modes and Functional Guilds
4. Discussion
4.1. Root-Associated Fungal Communities Are Related to the Environemntal Conditions Supporting Production of Dominant Truffle Species
4.2. Root-Associated Fungal Communities in Balkan Riparian Forests Are Charcaterised by Core and Forest Type Specific Genera
4.3. Dominance of Saprotrophs and Symbiotrophs across the Seasons in Root-Associated Fungal Communities in the Studied Truffle-Producing Forests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahram, M.; Peay, K.G.; Tedersoo, L. Local-scale biogeography and spatiotemporal variability in communities of mycorrhizal fungi. New Phytol. 2015, 205, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Opik, M.; Zobel, M.; Vasar, M.; Metsis, M.; Moora, M. Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS ONE 2012, 7, e41938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.A.; Nara, K.; Hogetsu, T. Host effects on ectomycorrhizal fungal communities: Insight from eight host species in mixed conifer-broadleaf forests. New Phytol. 2007, 174, 430–440. [Google Scholar] [CrossRef]
- Jumpponen, A.; Jones, K.L.; Mattox, D.; Yaege, C. Massively parallel 454-sequencing of fungal communities in Quercus spp. ectomycorrhizas indicates seasonal dynamics in urban and rural sites. Mol. Ecol. 2010, 19, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Opik, M.; Metsis, M.; Daniell, T.J.; Zobel, M.; Moora, M. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 2009, 184, 424–437. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef] [Green Version]
- Albornoz, F.E.; Teste, F.P.; Lambers, H.; Bunce, M.; Murray, D.C.; White, N.E.; Laliberte, E. Changes in ectomycorrhizal fungal community composition and declining diversity along a 2-million-year soil chronosequence. Mol. Ecol. 2016, 25, 4919–4929. [Google Scholar] [CrossRef]
- Goldmann, K.; Schroter, K.; Pena, R.; Schoning, I.; Schrumpf, M.; Buscot, F.; Polle, A.; Wubet, T. Divergent habitat filtering of root and soil fungal communities in temperate beech forests. Sci. Rep. 2016, 6, 31439. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Winston, G.C.; Goulden, M.L.; Treseder, K.K. Environmental filtering affects soil fungal community composition more than dispersal limitation at regional scales. Fungal Ecol. 2014, 12, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Wubet, T.; Christ, S.; Schoning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in soil fungal communities between European beech (Fagus sylvatica L.) Dominated forests are related to soil and understory vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- Koorem, K.; Gazol, A.; Opik, M.; Moora, M.; Saks, U.; Uibopuu, A.; Sober, V.; Zobel, M. Soil nutrient content influences the abundance of soil microbes but not plant biomass at the small-scale. PLoS ONE 2014, 9, e91998. [Google Scholar] [CrossRef] [Green Version]
- Santalahti, M.; Sun, H.; Jumpponen, A.; Pennanen, T.; Heinonsalo, J. Vertical and seasonal dynamics of fungal communities in boreal Scots pine forest soil. FEMS Microbiol. Ecol. 2016, 92, fiw170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroter, K.; Wemheuer, B.; Pena, R.; Schoning, I.; Ehbrecht, M.; Schall, P.; Ammer, C.; Daniel, R.; Polle, A. Assembly processes of trophic guilds in the root mycobiome of temperate forests. Mol. Ecol. 2019, 28, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.R.; Brunner, I.; Ashwood, F.; Bjarnadottir, B.; Bolger, T.; Børja, I.; Carnol, M.; Cudlin, P.; Dalsgaard, L.; Erktan, A. Belowground biodiversity relates positively to ecosystem services of European forests. Front. For. Glob. Chang. 2019, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Bohn, U.; Neuhäusl, R.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, T.; Schluter, H.; Weber, H. Karte der Natürlichen Vegetation Europas/Map of the Natural Vegetation of Europe; Maßstab/Scale 1:2,500,000; Landwirtschaftsverlag: Munster, Germany, 2003. [Google Scholar]
- Magyari, E.K.; Chapman, J.C.; Passmore, D.G.; Allen, J.R.M.; Huntley, J.P.; Huntley, B. Holocene persistence of wooded steppe in the Great Hungarian Plain. J. Biogeogr. 2010, 37, 915–935. [Google Scholar] [CrossRef]
- Purger, D.; Lengyel, A.; Kevey, B.; Lendvai, G.; Horvath, A.; Tomic, Z.; Csiky, J. Numerical classification of oak forests on loess in Hungary, Croatia and Serbia. Preslia 2014, 86, 47–66. [Google Scholar]
- Zólyomi, B. Magyarország természetes növénytakarója. [Map of the natural vegetation of Hungary.]. Nemzeti Atlasz Kartográfia Vállalat Budapest 1989, 89. [Google Scholar]
- Cestaric, D.; Skvorc, Z.; Franjic, J.; Sever, K.; Krstonosic, D. Forest plant community changes in the Spava lowland area (E Croatia). Plant Biosyst. 2017, 151, 584–597. [Google Scholar] [CrossRef]
- Deiller, A.F.; Walter, J.M.N.; Tremolieres, M. Effects of flood interruption on species richness, diversity and floristic composition of woody regeneration in the upper Rhine alluvial hardwood forest. Regul. River 2001, 17, 393–405. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. The value of wetlands: Importance of scale and landscape setting. Ecol. Econ. 2000, 35, 25–33. [Google Scholar] [CrossRef]
- Schnitzler, A. European Alluvial hardwood forests of large floodplains. J. Biogeogr. 1994, 21, 605–623. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Marjanovic, Z.; Grebenc, T.; Markovic, M.; Glisic, A.; Milenkovic, M. Ecological specificities and molecular diversity of truffles (genus Tuber) originating from mid-west of the Balkan Peninsula. Sydowia 2010, 62, 67–87. [Google Scholar]
- Marjanovic, Z.; Glisic, A.; Mutavdzic, D.; Saljnikov, E.; Bragato, G. Ecosystems supporting Tuber magnatum Pico production in Serbia experience specific soil environment seasonality that may facilitate truffle lifecycle completion. Appl. Soil. Ecol. 2015, 95, 179–190. [Google Scholar] [CrossRef]
- Bencivenga, M.; Granetti, B. Ricerca comparativa sulle esigenze ecologiche di Tuber magnatum Pico e Tuber melanosporum Vitt. dell’Italia Centrale. Ann. Della Fac. Di Agraria. Univ. Degli Studi Di Perugia 1988, 42, 861–872. [Google Scholar]
- Iotti, M.; Leonardi, P.; Vitali, G.; Zambonelli, A. Effect of summer soil moisture and temperature on the vertical distribution of Tuber magnatum mycelium in soil. Biol. Fertil. Soils 2018, 54, 707–716. [Google Scholar] [CrossRef]
- Bragato, G.; Marjanović, Ž.S. Soil characteristics for Tuber magnatum. In True Truffle (Tuber spp.) in the World; Springer: Cham, Switzerland, 2016; pp. 191–209. [Google Scholar]
- Bragato, G.; Sladonja, B.; Peršurić, Đ. The soil environment for Tuber magnatum growth in Motovun forest, Istria. Nat. Croat. Period. Musei Hist. Nat. Croat. 2004, 13, 171–185. [Google Scholar]
- Bragato, G.; Vignozzi, N.; Pellegrini, S.; Sladonja, B. Physical characteristics of the soil environment suitable for Tuber magnatum production in fluvial landscapes. Plant Soil 2010, 329, 51–63. [Google Scholar] [CrossRef]
- Iotti, M.; Leonardi, M.; Lancellotti, E.; Salerni, E.; Oddis, M.; Leonardi, P.; Perini, C.; Pacioni, G.; Zambonelli, A. Spatio-temporal dynamic of Tuber magnatum mycelium in natural truffle grounds. PLoS ONE 2014, 9, e115921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belfiori, B.; Riccioni, C.; Tempesta, S.; Pasqualetti, M.; Paolocci, F.; Rubini, A. Comparison of ectomycorrhizal communities in natural and cultivated Tuber melanosporum truffle grounds. FEMS Microbiol. Ecol. 2012, 81, 547–561. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Raggi, L.; Albertini, E.; Csorbai, A.G.; Donnini, D. Assessment of ectomycorrhizal biodiversity in Tuber macrosporum productive sites. Mycorrhiza 2014, 24, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Benucci, G.M.N.; Raggi, L.; Albertini, E.; Grebenc, T.; Bencivenga, M.; Falcinelli, M.; Di Massimo, G. Ectomycorrhizal communities in a productive Tuber aestivum Vittad. orchard: Composition, host influence and species replacement. FEMS Microbiol. Ecol. 2011, 76, 170–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iotti, M.; Lancellotti, E.; Hall, I.; Zambonelli, A. The ectomycorrhizal community in natural Tuber borchii grounds. FEMS Microbiol. Ecol. 2010, 72, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, M.; Iotti, M.; Oddis, M.; Lalli, G.; Pacioni, G.; Leonardi, P.; Maccherini, S.; Perini, C.; Salerni, E.; Zambonelli, A. Assessment of ectomycorrhizal fungal communities in the natural habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 2013, 23, 349–358. [Google Scholar] [CrossRef]
- Murat, C.; Vizzini, A.; Bonfante, P.; Mello, A. Morphological and molecular typing of the below-ground fungal community in a natural Tuber magnatum truffle-ground. FEMS Microbiol. Lett. 2005, 245, 307–313. [Google Scholar] [CrossRef]
- Gryndler, M.; Hrselova, H.; Soukupova, L.; Streiblova, E.; Valda, S.; Borovicka, J.; Gryndlerova, H.; Gazo, J.; Miko, M. Detection of summer truffle (Tuber aestivum Vittad.) in ectomycorrhizae and in soil using specific primers. FEMS Microbiol. Lett. 2011, 318, 84–91. [Google Scholar] [CrossRef]
- Bertini, L.; Rossi, I.; Zambonelli, A.; Amicucci, A.; Sacchi, A.; Cecchini, M.; Gregori, G.; Stocchi, V. Molecular identification of Tuber magnatum ectomycorrhizae in the field. Microbiol. Res. 2006, 161, 59–64. [Google Scholar] [CrossRef]
- Zampieri, E.; Murat, C.; Cagnasso, M.; Bonfante, P.; Mello, A. Soil analysis reveals the presence of an extended mycelial network in a Tuber magnatum truffle-ground. FEMS Microbiol. Ecol. 2010, 71, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, S.; Carevic, I.; Kostadinov, S.; Novkovic, I.; Abolmasov, B.; Milojkovic, B.; Simic, D. Landslide Susceptibility Zonation in the Kolubara River Basin (Western Serbia)—Analysis of Input Data. Carpath. J. Earth Environ. 2012, 7, 37–47. [Google Scholar]
- Enger, H.; Riehm, H. Die ammoniumlaktatessigsäure-methode zur bestimmung der leichtlöslichen phosphorsäure in karbonathaltigen böden. Agrochimica 1958, 3, 49–65. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter; Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Ihrmark, K.; Bodeker, I.T.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandstrom-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press Inc.: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Boyer, F.; Mercier, C.; Bonin, A.; Le Bras, Y.; Taberlet, P.; Coissac, E. OBITOOLS: A unix-inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 2016, 16, 176–182. [Google Scholar] [CrossRef]
- Nawaz, A.; Purahong, W.; Herrmann, M.; Kusel, K.; Buscot, F.; Wubet, T. DNA- and RNA-derived fungal communities in subsurface aquifers only partly overlap but react similarly to environmental factors. Microorganisms 2019, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Purahong, W.; Lehmann, R.; Herrmann, M.; Totsche, K.U.; Kusel, K.; Wubet, T.; Buscot, F. First insights into the living groundwater mycobiome of the terrestrial biogeosphere. Water Res. 2018, 145, 50–61. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ. 2016, 4, e2584. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glockner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; De Wit, P.; Sanchez-Garcia, M.; Ebersberger, I.; de Sousa, F.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Kunin, V.; Engelbrektson, A.; Ochman, H.; Hugenholtz, P. Wrinkles in the rare biosphere: Pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 2010, 12, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.3-1; World Agroforestry: Nairobi, Kenya, 2015. [Google Scholar]
- Bertini, L.; Agostini, D.; Potenza, L.; Rossi, I.; Zeppa, S.; Zambonelli, A.; Stocchi, V. Molecular markers for the identification of the ectomycorrhizal fungus Tuber borchii. New Phytol. 1998, 139, 565–570. [Google Scholar] [CrossRef]
- Tedersoo, L.; Jairus, T.; Horton, B.M.; Abarenkov, K.; Suvi, T.; Saar, I.; Koljalg, U. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol. 2008, 180, 479–490. [Google Scholar] [CrossRef]
- Smith, M.E.; Douhan, G.W.; Fremier, A.K.; Rizzo, D.M. Are true multihost fungi the exception or the rule? Dominant ectomycorrhizal fungi on Pinus sabiniana differ from those on co-occurring Quercus species. New Phytol. 2009, 182, 295–299. [Google Scholar] [CrossRef]
- Glassman, S.I.; Wang, I.J.; Bruns, T.D. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Mol. Ecol. 2017, 26, 6960–6973. [Google Scholar] [CrossRef] [Green Version]
- Molinier, V.; Murat, C.; Baltensweiler, A.; Buntgen, U.; Martin, F.; Meier, B.; Moser, B.; Sproll, L.; Stobbe, U.; Tegel, W.; et al. Fine-scale genetic structure of natural Tuber aestivum sites in southern Germany. Mycorrhiza 2016, 26, 895–907. [Google Scholar] [CrossRef]
- Kues, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biot. 2000, 54, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Parlade, J.; De la Varga, H.; De Miguel, A.M.; Saez, R.; Pera, J. Quantification of extraradical mycelium of Tuber melanosporum in soils from truffle orchards in northern Spain. Mycorrhiza 2013, 23, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Queralt, M.; Parlade, J.; Pera, J.; de Miguel, A.M. Seasonal dynamics of extraradical mycelium and mycorrhizas in a black truffle (Tuber melanosporum) plantation. Mycorrhiza 2017, 27, 565–576. [Google Scholar] [CrossRef]
- Todesco, F.; Belmondo, S.; Guignet, Y.; Laurent, L.; Fizzala, S.; Le Tacon, F.; Murat, C. Soil temperature and hydric potential influences the monthly variations of soil Tuber aestivum DNA in a highly productive orchard. Sci. Rep. 2019, 9, 12964. [Google Scholar] [CrossRef] [PubMed]
- Mangeot-Peter, L.; Tschaplinski, T.J.; Engle, N.L.; Veneault-Fourrey, C.; Martin, F.; Deveau, A. Impacts of soil microbiome variations on root colonization by fungi and bacteria and on the metabolome of Populus tremula xalba. Phytobiomes J. 2020, 4, 142–155. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, A.M.; Agueda, B.; Sanchez, S.; Parlade, J. Ectomycorrhizal fungus diversity and community structure with natural and cultivated truffle hosts: Applying lessons learned to future truffle culture. Mycorrhiza 2014, 24 (Suppl. 1), S5–S18. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L.; Klironomos, J.N. Using ecological network theory to evaluate the causes and consequences of arbuscular mycorrhizal community structure. New Phytol. 2012, 194, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Navarro, A.; Segarra-Moragues, J.G.; Valiente-Banuet, A.; Verdu, M. The network structure of plant-arbuscular mycorrhizal fungi. New Phytol. 2012, 194, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Tedersoo, L.; Nilsson, R.H.; Abarenkov, K.; Jairus, T.; Sadam, A.; Saar, I.; Bahram, M.; Bechem, E.; Chuyong, G.; Koljalg, U. 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol. 2010, 188, 291–301. [Google Scholar] [CrossRef]
- Peay, K.G.; Bruns, T.D.; Kennedy, P.G.; Bergemann, S.E.; Garbelotto, M. A strong species-area relationship for eukaryotic soil microbes: Island size matters for ectomycorrhizal fungi. Ecol. Lett. 2007, 10, 470–480. [Google Scholar] [CrossRef]
- Bonito, G.M.; Smith, M.E. General systematic position of the truffles: Evolutionary theories. In True Truffle (Tuber spp.) in the World; Springer: Cham, Switzerland, 2016; pp. 3–18. [Google Scholar]
- Hilszczanska, D.; Szmidla, H.; Sikora, K.; Rosa-Gruszecka, A. Soil properties conducive to the formation of Tuber aestivum vitt. fruiting bodies. Pol. J. Environ. Stud. 2019, 28, 1713–1718. [Google Scholar] [CrossRef]
- Weden, C.; Chevalier, G.; Danell, E. Tuber aestivum (syn. T-uncinatum) biotopes and their history on Gotland, Sweden. Mycol. Res. 2004, 108, 304–310. [Google Scholar] [CrossRef]
- Li, Q.; Yan, L.J.; Ye, L.; Zhou, J.; Zhang, B.; Peng, W.H.; Zhang, X.P.; Li, X.L. Chinese black truffle (Tuber indicum) alters the ectomycorrhizosphere and endoectomycosphere microbiome and metabolic profiles of the host tree Quercus aliena. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoen, E.; Harder, C.B.; Kauserud, H.; Botnen, S.S.; Vik, U.; Taylor, A.F.S.; Menkis, A.; Skrede, I. In vitro evidence of root colonization suggests ecological versatility in the genus Mycena. New Phytol. 2020, 227, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonito, G.; Benucci, G.M.N.; Hameed, K.; Weighill, D.; Jones, P.; Chen, K.H.; Jacobson, D.; Schadt, C.; Vilgalys, R. Fungal-Bacterial Networks in the Populus Rhizobiome Are Impacted by Soil Properties and Host Genotype. Front. Microbiol. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River. Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Bobinac, M.; Čater, M. Ekologija i Obnova Higrofilnih Lužnjakovih šuma Ravnog Srema; Hrvatski Šumarski Institute: Jastrebarsko, Croatia, 2011. [Google Scholar]
- Samaritani, E.; Mitchell, E.A.D.; Rich, J.; Shrestha, J.; Fournier, B.; Frey, B. Soil bacterial communities and ecosystem functioning change more strongly with season than habitat in a restored floodplain. Appl. Soil. Ecol. 2017, 112, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Geml, J.; Laursen, G.A.; Herriott, I.C.; McFarland, J.M.; Booth, M.G.; Lennon, N.; Chad Nusbaum, H.; Lee Taylor, D. Phylogenetic and ecological analyses of soil and sporocarp DNA sequences reveal high diversity and strong habitat partitioning in the boreal ectomycorrhizal genus Russula (Russulales; Basidiomycota). New Phytol. 2010, 187, 494–507. [Google Scholar] [CrossRef]
- Stielow, B.; Bratek, Z.; Orczan, A.K.; Rudnoy, S.; Hensel, G.; Hoffmann, P.; Klenk, H.P.; Goker, M. Species delimitation in taxonomically difficult fungi: The case of Hymenogaster. PLoS ONE 2011, 6, e15614. [Google Scholar] [CrossRef]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Chen, C.; Verkley, G.J.; Sun, G.; Groenewald, J.Z.; Crous, P.W. Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biol. 2016, 120, 1291–1322. [Google Scholar] [CrossRef] [Green Version]
- Bahnmann, B.; Masinova, T.; Halvorsen, R.; Davey, M.L.; Sedlak, P.; Michal; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Kolarikova, Z.; Kohout, P.; Kruger, C.; Janouskova, M.; Mrnka, L.; Rydlova, J. Root-associated fungal communities along a primary succession on a mine spoil: Distinct ecological guilds assemble differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Veach, A.M.; Stokes, C.E.; Knoepp, J.; Jumpponen, A.; Baird, R. Fungal communities and functional guilds shift along an elevational gradient in the Southern Appalachian mountains. Microb. Ecol. 2018, 76, 156–168. [Google Scholar] [CrossRef]
- Goldmann, K.; Schoning, I.; Buscot, F.; Wubet, T. Forest management type influences diversity and community composition of soil fungi across temperate forest ecosystems. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Buee, M.; Vairelles, D.; Garbaye, J. Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus silvatica) forest subjected to two thinning regimes. Mycorrhiza 2005, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Courty, P.E.; Franc, A.; Garbaye, J. Temporal and functional pattern of secreted enzyme activities in an ectomycorrhizal community. Soil Biol. Biochem. 2010, 42, 2022–2025. [Google Scholar] [CrossRef]
- Koide, R.T.; Shumway, D.L.; Xu, B.; Sharda, J.N. On temporal partitioning of a community of ectomycorrhizal fungi. New Phytol. 2007, 174, 420–429. [Google Scholar] [CrossRef]
- Voriskova, J.; Brabcova, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef]
- Wallander, H.; Nilsson, L.O.; Hagerberg, D.; Baath, E. Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol. 2001, 151, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Högberg, M.N.; Briones, M.J.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Midwood, A.J.; Thornton, B.; Hurry, V.; Linder, S.; Näsholm, T. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 2010, 187, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Baum, C.; Makeschin, F. Effects of nitrogen and phosphorus fertilization on mycorrhizal formation of two poplar clones (Populus trichocarpa and P-tremula x tremuloides). J. Plant Nutr. Soil Sci. 2000, 163, 491–497. [Google Scholar] [CrossRef]
- Lodge, D.J. The influence of soil-moisture and flooding on formation of Va-Endomycorrhizae and Ectomycorrhizae in Populus and Salix. Plant Soil 1989, 117, 243–253. [Google Scholar] [CrossRef]
- Herzog, C.; Peter, M.; Pritsch, K.; Gunthardt-Goerg, M.S.; Egli, S. Drought and air warming affects abundance and exoenzyme profiles of Cenococcum geophilum associated with Quercus robur, Q. petraea and Q. pubescens. Plant Biol. 2013, 15, 230–237. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [Green Version]
- Podila, G.K.; Sreedasyam, A.; Muratet, M.A. Populus rhizosphere and the ectomycorrhizal interactome. Crit. Rev. Plant. Sci. 2009, 28, 359–367. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Laliberte, E. Soil Development and Nutrient Availability Along a 2 Million-Year Coastal Dune Chronosequence Under Species-Rich Mediterranean Shrubland in Southwestern Australia. Ecosystems 2015, 18, 287–309. [Google Scholar] [CrossRef]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Upson, R.; Read, D.J.; Newsham, K.K. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 2009, 20, 1–11. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.R.; Finlay, R.D.; Stenlid, J.; Vasaitis, R.; Menkis, A. Growing evidence for facultative biotrophy in saprotrophic fungi: Data from microcosm tests with 201 species of wood-decay basidiomycetes. New Phytol. 2017, 215, 747–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soudzilovskaia, N.A.; Douma, J.C.; Akhmetzhanova, A.A.; van Bodegom, P.M.; Cornwell, W.K.; Moens, E.J.; Treseder, K.K.; Tibbett, M.; Wang, Y.P.; Cornelissen, J.H.C. Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob. Ecol. Biogeogr. 2015, 24, 371–382. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T.; Rohr, J.R.; Aldrich-Wolfe, L.; Morton, J.B. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J. Ecol. 2007, 95, 95–105. [Google Scholar] [CrossRef]
- Toljander, J.F.; Eberhardt, U.; Toljander, Y.K.; Paul, L.R.; Taylor, A.F.S. Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol. 2006, 170, 873–883. [Google Scholar] [CrossRef]
- Grunfeld, L.; Wulf, M.; Rillig, M.C.; Manntschke, A.; Veresoglou, S.D. Neighbours of arbuscular-mycorrhiza associating trees are colonized more extensively by arbuscular mycorrhizal fungi than their conspecifics in ectomycorrhiza dominated stands. New Phytol. 2020, 227, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Veresoglou, S.D.; Wulf, M.; Rillig, M.C. Facilitation between woody and herbaceous plants that associate with arbuscular mycorrhizal fungi in temperate European forests. Ecol. Evol. 2017, 7, 1181–1189. [Google Scholar] [CrossRef]
- Bueno, C.G.; Moora, M.; Gerz, M.; Davison, J.; Öpik, M.; Pärtel, M.; Helm, A.; Ronk, A.; Kühn, I.; Zobel, M. Plant mycorrhizal status, but not type, shifts with latitude and elevation in Europe. Glob. Ecol. Biogeogr. 2017, 26, 690–699. [Google Scholar] [CrossRef]
- Van der Heijden, E.W.; Kuyper, T.W. Does origin of mycorrhizal fungus or mycorrhizal plant influence effectiveness of the mycorrhizal symbiosis? Plant Soil 2001, 230, 161–174. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Hobbie, E.A.; Fahey, T.J. Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. New Phytol. 2002, 154, 219–231. [Google Scholar] [CrossRef]







| Sample | pH | SOM (%) | Available | Available (K2O) | CaCO3 (%) | Ctot (%) | Ntot (%) | C/N | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KCl | H2O | P2O5/jun (mg/100 g) | P2O5/aug (mg/100 g) | P2O5/nov (mg/100 g) | |||||||
| WTF1 | 7.30 | 8.00 | 2.29 | 10.18 | 8.20 | 8.45 | 12.61 | 5.44 | 1.52 | 0.08 | 20.73 |
| WTF2 | 7.50 | 8.10 | 2.27 | 7.46 | 9.45 | 9.58 | 17.12 | 6.49 | 1.83 | 0.09 | 19.71 |
| WTF3 | 7.60 | 8.10 | 1.05 | 11.98 | 7.46 | 11.53 | 9.45 | 5.02 | 1.03 | 0.04 | 22.80 |
| MTF1 | 5.60 | 6.40 | 3.99 | 9.60 | 20.81 | 3.02 | 21.07 | - | 2.16 | 0.23 | 9.39 |
| MTF2 | 5.80 | 6.60 | 3.81 | 10.93 | 19.44 | 3.32 | 20.21 | - | 2.41 | 0.24 | 10.02 |
| MTF3 | 6.30 | 7.00 | 4.78 | 20.95 | 21.86 | 14.89 | 31.82 | - | 2.91 | 0.29 | 10.09 |
| BTF1 | 4.00 | 5.00 | 4.90 | 6.48 | 13.05 | 5.22 | 24.36 | - | 2.85 | 0.28 | 10.21 |
| BTF2 | 3.80 | 5.10 | 4.90 | 5.38 | 12.46 | 4.95 | 11.03 | - | 2.28 | 0.23 | 10.08 |
| BTF3 | 3.90 | 4.70 | 5.00 | 4.47 | 8.66 | 3.82 | 20.71 | - | 2.70 | 0.25 | 10.89 |
| Variable | R2 | p |
|---|---|---|
| clay (%) | 0.5737 | 0.001 *** |
| silt (%) | 0.8533 | 0.001 *** |
| sand (%) | 0.7169 | 0.001 *** |
| pH | 0.8677 | 0.001 *** |
| OM (%) | 0.7713 | 0.001 *** |
| K2O | 0.1964 | 0.083 |
| P2O5 | 0.2081 | 0.058 |
| Distance from river (m) | 0.8826 | 0.001 *** |
| Elevation (masl) | 0.7499 | 0.001 *** |
| N (%) | 0.7226 | 0.001 *** |
| C (%) | 0.5631 | 0.001 *** |
| C:N ratio | 0.7999 | 0.001 *** |
| Forest type | 0.5734 | 0.001 *** |
| Fungal Genera | R2 | p |
|---|---|---|
| Alternaria sp. | 0.432 | 0.001 *** |
| Calycina sp. | 0.275 | 0.004 ** |
| Cladophialophora sp. | 0.288 | 0.006 ** |
| Cladorrhinum sp. | 0.288 | 0.008 ** |
| Cladosporium sp. | 0.341 | 0.004 ** |
| Fusarium sp. | 0.343 | 0.006 ** |
| Gibberella sp. | 0.364 | 0.001 *** |
| Humaria sp. | 0.294 | 0.01 ** |
| Humicola sp. | 0.375 | 0.007 ** |
| Hydnotrya sp. | 0.278 | 0.01 ** |
| Hymenogaster sp. | 0.253 | 0.006 ** |
| Hymenoscyphus sp. | 0.349 | 0.005 ** |
| Mycoarthris sp. | 0.503 | 0.001 *** |
| Olpidium sp. | 0.364 | 0.002 ** |
| Pezicula sp. | 0.287 | 0.009 ** |
| Phialophora sp. | 0.283 | 0.004 ** |
| Pyrenochaeta sp. | 0.245 | 0.01 ** |
| Russula sp. | 0.347 | 0.005 ** |
| Subulicystidium sp. | 0.571 | 0.001 *** |
| White Truffle-Producing Forest | Mixed Truffle-Producing Forest | Black Truffle-Producing Forest | |||
|---|---|---|---|---|---|
| Ascochyta sp. | 69.78% of total sequences | Ascochyta sp. | 32.45% of total sequences | Ascochyta sp. | 39.55% of total sequences |
| Exophiala sp. | Exophiala sp. | Exophiala sp. | |||
| Ilyonectria sp. | Ilyonectria sp. | Ilyonectria sp. | |||
| Inocybe sp. | Inocybe sp. | Inocybe sp. | |||
| Tetracladium sp. | Tetracladium sp. | Tetracladium sp. | |||
| Tomentella sp. | Tomentella sp. | Tomentella sp. | |||
| Alternaria sp. | Cladophialophora sp. | Cladophialophora sp. | |||
| Ascobolus sp. | Humicola sp. | Humicola sp. | |||
| Calycina sp. | Hyphodiscus sp. | Hyphodiscus sp. | |||
| Cistella sp. | Mortierella sp. | Mortierella sp. | |||
| Cyphellophora sp. | Mycena sp. | Mycena sp. | |||
| Fusarium sp. | Mycenella sp. | Mycenella sp. | |||
| Gibberella sp. | Penicillium sp. | Penicillium sp. | |||
| Hebeloma sp. | Russula sp. | Russula sp. | |||
| Hymenoscyphus sp. | Sebacina sp. | Sebacina sp. | |||
| Microdochium sp. | Athelopsis sp. | ||||
| Mycoarthris sp. | Cenococcum sp. | ||||
| Paraphoma sp. | Entoloma sp. | ||||
| Plectosphaerella sp. | Herpotrichia sp. | ||||
| Plenodomus sp. | Humaria sp. | ||||
| Podospora sp. | Menispora sp. | ||||
| Pyrenochaeta sp. | Oidiodendron sp. | ||||
| Rhizoglomus sp. | Pezicula sp. | ||||
| Schizothecium sp. | Phallus sp. | ||||
| Scleropezicula sp. | Saitozyma sp. | ||||
| Subulicystidium sp. | Trechispora sp. | ||||
| Tuber sp. | Trichoderma sp. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marjanović, Ž.; Nawaz, A.; Stevanović, K.; Saljnikov, E.; Maček, I.; Oehl, F.; Wubet, T. Root-Associated Mycobiome Differentiate between Habitats Supporting Production of Different Truffle Species in Serbian Riparian Forests. Microorganisms 2020, 8, 1331. https://doi.org/10.3390/microorganisms8091331
Marjanović Ž, Nawaz A, Stevanović K, Saljnikov E, Maček I, Oehl F, Wubet T. Root-Associated Mycobiome Differentiate between Habitats Supporting Production of Different Truffle Species in Serbian Riparian Forests. Microorganisms. 2020; 8(9):1331. https://doi.org/10.3390/microorganisms8091331
Chicago/Turabian StyleMarjanović, Žaklina, Ali Nawaz, Katarina Stevanović, Elmira Saljnikov, Irena Maček, Fritz Oehl, and Tesfaye Wubet. 2020. "Root-Associated Mycobiome Differentiate between Habitats Supporting Production of Different Truffle Species in Serbian Riparian Forests" Microorganisms 8, no. 9: 1331. https://doi.org/10.3390/microorganisms8091331