Abstract
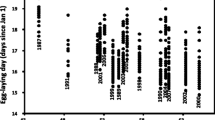
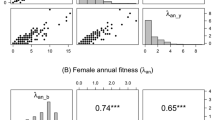
The conservation of endangered species critically depends on the understanding to which degree short-term fitness and long-term trends are affected by intrinsic local conditions and external global dynamics. However, studies combining long-term demographic data with population level analyses of site conditions, genetic variation, and reproduction as well as with climatic data are still rare. Here we studied the endangered orchid Anacamptis morio, representative for species with a sub-mediterranean distribution. For populations at the northern range edge, we combined long-term monitoring data (1977–2010) with climatic data and analyzed reproductive fitness components, genetic variation, and abiotic site conditions. Reproduction was generally low as expected from the deceptive pollination system, and was positively influenced by population size and xerothermic site quality. The majority of populations showed a positive population trend, which was paralleled by an increase in spring temperature and positively affected by site quality. High levels of genetic variation were found in the populations which were at gene flow-drift equilibrium. A. morio may profit from increasing spring temperatures because of increased reproductive output. Nevertheless, whether climate change results in fitness increase or not may depend on the maintenance and provision of optimal site quality, i.e., xerothermic and nutrient poor conditions.



Similar content being viewed by others
References
Ågren J (1996) Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria. Ecology 77:1779–1790
Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Nat Acad Sci 108:20645–20649
Bernhardt P, Edens-Meier R (2010) What we think we know vs. what we need to know about orchid pollination and conservation: Cypripedium L. as a model lineage. Bot Rev 76:204–219
Bizoux JP, Dainou K, Raspe O, Lutts S, Mahy G (2008) Fitness and genetic variation of Viola calaminaria, an endemic metallophyte: implications of population structure and history. Plant Biol 10:684–693
Böhnert W, Hecht G, Stapperfenne H-J (1986) Orchideen des Bezirkes Halle. einst und jetzt, Dessau
Bruelheide H, Lieberum K (2001) Experimental tests for determining the causes of the altitudinal distribution of Meum athamanticum Jacq. in the Harz Mountains. Flora 196:227–241
Chesson P, Huntly N (1989) Short-term instabilities and long-term community dynamics. Trends Ecol Evol 4:293–298
Coart E, Van Glabeke S, Petit RJ, Van Bockstaele E, Roldan-Ruiz I (2005) Range wide versus local patterns of genetic diversity in hornbeam (Carpinus betulus L.). Conserv Genet 6:259–273
Csergö AM, Molnar E, Garcia MB (2011) Dynamics of isolated Saponaria bellidifolia Sm. populations at northern range periphery. Popul Ecol 53:393–403
Dupré C, Diekmann M (1998) Prediction of occurrence of vascular plants in deciduous forests of South Sweden by means of Ellenberg indicator values. Appl Veg Sci 1:139–150
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa. Goltze, Göttingen
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size - implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc 81:117–142
Fay MF, Rankou H (2010) 670. Anacamptis morio. Curtis’s Bot Mag 27:100–108
Feehan J, Harley M, van Minnen J (2009) Climate change in Europe. 1. Impact on terrestrial ecosystems and biodiversity. A review. Agron Sustain Dev 29:409–421
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63:151–162
Hedhly A, Hormaza JI, Herrero M (2009) Global warming and sexual plant reproduction. Trends Plant Sci 14:30–36
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
Humbert J-Y, Scott MillsL, Horne JS, Dennis B (2009) A better way to estimate population trends. Oikos 118:1940–1946
Hutchison DW, Templeton AR (1999) Correlation of pairwise genetic and geographic distance measures: Inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution 53:1898–1914
Jacquemyn H, Brys R, Hermy M, Willems JH (2005) Does nectar reward affect rarity and extinction probabilities of orchid species? An assessment using historical records from Belgium and the Netherlands. Biol Conserv 121:257
Jacquemyn H, Brys R, Adriaens D, Honnay O, Roldan-Ruiz I (2009) Effects of population size and forest management on genetic diversity and structure of the tuberous orchid Orchis mascula. Conserv Genet 10:161–168
Jersakova J, Kindlmann P (1998) Patterns of pollinator-generated fruit set in Orchis morio (Orchidaceae). Folia Geobot 33:377–390
Jersakova J, Malinova T (2007) Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytol 176:237–241
Jersakova J, Kindlmann P, Stritesky M (2002) Population dynamics of Orchis morio in the Czech Republic under human influence. In: Kindlmann P, Willems JH, Whigham DF (eds) Trends and fluctuations and underlying mechanisms in terrestrial orchid populations. Backhuys, Leiden, pp 224–299
Jersakova J, Johnson SD, Kindlmann P (2006) Mechanisms and evolution of deceptive pollination in orchids. Biol Rev Camb Philos Soc 81:219–235
Johnson SD, Peter CI, Nilsson LA, Agren J (2003) Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84:2919–2927
Jongejans E, Jorritsma-Wienk LD, Becker U, Dostal P, Milden M, de Kroon H (2010) Region versus site variation in the population dynamics of three short-lived perennials. J Ecol 98:279–289
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman TL (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Kretzschmar H, Eccarius W, Dietrich H (2007) The orchid genera Anacamptis, Orchis and Neotinea. Phylogeny, taxonomy, morphology, biology, distribution, ecology and hybridisation. EchinoMedia Verlag, Bürgel
Kull T, Hutchings MJ (2006) A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom. Biol Conserv 129:31
Lauterbach D, Ristow M, Gemeinholzer B (2011) Genetic population structure, fitness variation and the importance of population history in remnant populations of the endangered plant Silene chlorantha (Willd.) Ehrh. (Caryophyllaceae). Plant Biol 13:667–677
Leimu R (2010) Habitat quality and population size as determinants of performance of two endangered hemiparasites. Ann Bot Fenn 47:1–13
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952
Lienert J, Fischer M, Schneller J, Diemer M (2002) Isozyme variability of the wetland specialist Swertia perennis (Gentianaceae) in relation to habitat size, isolation, and plant fitness. Am J Bot 89:801–811
Lynch M, Milligan BG (1994) Analysis of population genetic-structure with RAPD markers. Mol Ecol 3:91–99
Magnuson JJ (1990) Long-term ecological research and the invisible present. Bioscience 40:495–501
Menzel A, Estrella N, Fabian P (2001) Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Glob Change Biol 7:657–666
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83:1131–1145
Morris WF, Pfister CA, Tuljapurkar S, Haridas CV, Boggs CL, Boyce MS, Bruna EM, Church DR, Coulson T, Doak DF, Forsyth S, Gaillard JM, Horvitz CC, Kalisz S, Kendall BE, Knight TM, Lee CT, Menges ES (2008) Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89:19–25
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Neiland MRM, Wilcock CC (1998) Fruit set, nectar reward, and rarity in the Orchidaceae. Am J Bot 85:1657–1671
Nilsson LA (1984) Anthecology of Orchis morio (Orchidaceae) at its outpost in the north Nov. Act Reg Soc Sci Ups 3:167–179
Oostermeijer JGB, Luijten SH, den Nijs JCM (2003) Integrating demographic and genetic approaches in plant conservation. Biol Conserv 113:389–398
Peakall R, Beattie AJ (1996) Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentactulata. Evolution 50:2207–2220
Pfeifer M, Wiegand K, Heinrich W, Jetschke G (2006) Long-term demographic fluctuations in an orchid species driven by weather: implications for conservation planning. J Appl Ecol 43:313–324
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Robbirt KM, Davy AJ, Hutchings MJ, Roberts DL (2011) Validation of biological collections as a source of phenological data for use in climate change studies: a case study with the orchid Ophrys sphegodes. J Ecol 99:235–241
Rossi W, Corrias B, Arduino P, Cianchi R, Bullini L (1992) Gene variation and gene flow in Orchis morio (Orchidaceae) from Italy. Plant Syst Evol 179:43–58
Scacchi R, Deangelis G, Lanzara P (1990) Allozyme variation among and within 11 Orchis species (Fam Orchidaceae), with special reference to hybridizing aptitude. Genetica 81:143–150
Schaffers AP, Sýkora KV (2000) Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: a comparison with field measurements. J Veg Sci 11:225–244
Scopece G, Cozzolino S, Johnson SD, Schiestl FP (2010) Pollination efficiency and the evolution of specialized deceptive pollination systems. Am Nat 175:98–105
Silvertown J, Franco M, Pisanty I, Mendoza A (1993) Comparative plant demography—relative importance of life-cycle components to the finite rate of increase in woody and herbaceous perennials. J Ecol 81:465–476
Silvertown J, Wells DA, Gillman M, Dodd ME, Robertson H, Lakhani KH (1994) Short-term effects and long-term after-effects of fertilizer application on the flowering population of green-winged orchid Orchis morio. Biol Conserv 69:191
Smithson A (2006) Pollinator limitation and inbreeding depression in orchid species with and without nectar rewards. New Phytol 169:419–430
Sparks TH, Menzel A (2002) Observed changes in seasons: an overview. Int J Climatol 22:1715–1725
Steffan-Dewenter I, Münzenberg U, Tscharntke T (2001) Pollination, seed set and seed predation on a landscape scale. Proc R Soc Lond B Biol Sci 268:1685–1690
Vallius E, Salonen V, Kull T (2004) Factors of divergence in co-occurring varieties of Dactylorhiza incarnata (Orchidaceae). Plant Syst Evol 248:177–189
Vekemans X (2002) AFLP-SURV version 1.0. Distributed by the author. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles
Wells TCE, Rothery P, Cox R, Bamford S (1998) Flowering dynamics of Orchis morio L. and Herminium monorchis (L.) R.Br. at two sites in eastern England. Bot J Linn Soc 126:39–48
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Acknowledgments
We thank the working-groups for native orchids (AHO) of Saxony-Anhalt and Thuringia, for monitoring data, and for help with locating the populations. D. Frank made available species lists of many of the porphyry outcrops. We thank I. Geier and M. Herrmann for assistance with laboratory work. We thank J. Hanspach for help with statistical analyses and A. Kolb for helpful comments. This study was supported by the Federal Ministry of Education and Research (BMBF-FKz 01LC0618C) within the framework of BIOLOG-SUBICON (Successional Change and Biodiversity Conservation). Additionally, kind support was given by Helmholtz Impulse and Networking Fund through Helmholtz Interdisciplinary Graduate School for Environmental Research (HIGRADE).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hornemann, G., Michalski, S.G. & Durka, W. Short-term fitness and long-term population trends in the orchid Anacamptis morio . Plant Ecol 213, 1583–1595 (2012). https://doi.org/10.1007/s11258-012-0113-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-012-0113-6