Abstract
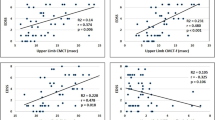
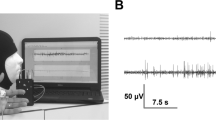
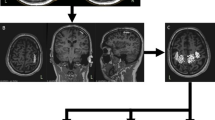
Introduction: Coexistent involvement of upper and lower motor neurons is a characteristic feature of amyotrophyc lateral syndrome (ALS) necessary for the diagnosis. Diagnosis of upper motor neuron involvement in ALS is based solely on clinical features, which may not be detected at the disease onset and in rare forms manifesting clinically as the pure lower motor neuron syndrome (LMNS). The main method of assessment of the functional state of the upper motor neuron in ALS is transcranial magnetic stimulation (TMS). It allows assessing the excitability of motor cortex, corticospinal tract function, and mapping of cortical representation of the muscles. In patients with ALS changes of various indicators demonstrating hyperexcitability as well as degenerative lesions of the motor cortex and the corticospinal tracts are recorded on TMS. Objective: to discuss changes in the TMS in patients with ALS, pathophysiological mechanisms of their formation and possible diagnostic value. Results: In 22 patients with LMNS, navigated TMS revealed disturbances of intracortical inhibition on paired stimulation and recording cortical silent period, increase of motor threshold in dominant hemisphere, decrease of the weighted area and reorganization of cortical representations of the hand muscles. Conclusion: The data obtained allow to consider navigated TMS as a promising technology for identifying upper motor neuron involvement in patients with ALS.


Similar content being viewed by others
REFERENCES
Zakharova, M.N., Brylev, L.V., Avdyunina, I.A., et al., Amyotrophic lateral sclerosis, in Nevrologiya. Natsional’noe rukovodstvo (Neurology: National Guide), Gusev, E.I., Konovalov, A.N., and Skvortsova, V.I., Eds., Moscow: GEOTAR-Media, 2018, no. 1, pp. 644–662.
Brooks, B.R., Miller, R.G., Swash, M., et al., El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis, Amyotrophic Lateral Scler. Frontotemporal Degener., 2000, vol. 1, pp. 293–299.
de Carvalho, M., Dengler, R., Eisen, A., et al., Electrodiagnostic criteria for diagnosis of ALS, Clin. Neurophysiol., 2008, vol. 119, pp. 497–503.https://doi.org/10.1016/j.clinph.2007.09.143
Swash, M., Why are upper motor neuron signs difficult to elicit in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry, 2012, vol. 83, pp. 659–662. https://doi.org/10.1136/jnnp-2012-302315
Huynh, W., Simon, N.G., Grosskreutz, J., et al., Assessment of the upper motor neuron in amyotrophic lateral sclerosis, Clin. Neurophysiol., 2016, vol. 127, pp. 2643–2660. https://doi.org/10.1016/j.clinph.2016.04.025
Ince, P.G., Evans, J., Knopp, M., et al., Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS, Neurology, 2003, vol. 60, pp. 1252–1258.
Liewluck, T. and Saperstein, D.S., Progressive muscular atrophy, Neurol. Clin., 2015, vol. 33, pp. 761–773. https://doi.org/10.1016/j.ncl.2015.07.005
Al-Chalabi, A., Hardiman, O., Kiernan, M.C., et al., Amyotrophic lateral sclerosis: moving towards a new classification system, Lancet Neurol., 2016, vol. 15, pp. 1182–1194. https://doi.org/10.1016/S1474-4422(16)30199-5
Swinnen, B. and Robberecht, W., The phenotypic variability of amyotrophic lateral sclerosis, Nat. Rev. Neurol., 2014, vol. 10, pp. 661–670. https://doi.org/10.1038/nrneurol.2014.184
Ludolph, A., Drory, V., Hardiman, O., et al., A revision of the El Escorial criteria 2015, Amyotrophic Lateral Scler. Frontotemporal Degener., 2015, vol. 16, pp. 291–292. https://doi.org/10.3109/21678421.2015.1049183
Bakulin, I.S., Zakroishchikova, I.V., Suponeva, N.A., and Zakharova, M.N., Amyotrophic lateral sclerosis: clinical heterogeneity and approaches to classification, Nervno-Myshechnye Bolezni, 2017, vol. 7, no. 3, pp. 10–20. https://doi.org/10.17650/2222-8721-2017-7-3-10-20
Garg, N., Park, S.B., Vucic, S., et al., Differentiating lower motor neuron syndromes, J. Neurol. Neurosurg. Psychiatry, 2017, vol. 88, pp. 474–483. https://doi.org/10.1136/jnnp-2016-313526
Sanderson, A.B., Arnold, W.D., Elsheikh, B., and Kissel, J.T., The clinical spectrum of isolated peripheral motor dysfunction, Muscle Nerve, 2015, vol. 51, pp. 358–362. https://doi.org/10.1002/mus.24326
Wijesekera, L.C., Mathers, S., Talman, P., et al., Natural history and clinical features of the flail arm and flail leg ALS variants, Neurology, 2009, vol. 72, pp. 1087–1094. https://doi.org/10.1212/01.wnl.0000345041.83406.a2
Hübers, A., Hildebrandt, V., Petri, S., et al., Clinical features and differential diagnosis of flail arm syndrome, J. Neurol., 2016, vol. 263, pp. 390–395. https://doi.org/10.1007/s00415-015-7993-z
Visser, J., van den Berg-Vos, R.M., Franssen, H., et al., Mimic syndromes in sporadic cases of progressive spinal muscular atrophy, Neurology, 2002, vol. 58, pp. 1593–1596.
Chiò, A., Pagani, M., Agosta, F., et al., Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes, Lancet Neurol., 2014, vol. 13, pp. 1228–1240. https://doi.org/10.1016/S1474-4422(14)70167-X
Pradat, P.F. and El Mendili, M.M., Neuroimaging to investigate multisystem involvement and provide biomarkers in amyotrophic lateral sclerosis, Biomed. Res. Int., 2014, vol. 2014, p. 467560. https://doi.org/10.1155/2014/467560
Grolez, G., Moreau, C., Danel-Brunaud, V., et al., The value of magnetic resonance imaging as a biomarker for amyotrophic lateral sclerosis: a systematic review, BMC Neurol., 2016, vol. 16, p. 155. https://doi.org/10.1186/s12883-016-0672-6
Bakulin, I.S., Chervyakov, A.V., Kremneva, E.I., et al., Structural and functional neuroimaging in amyotrophic lateral sclerosis, Ann. Klin. Eksp. Nevrol., 2017, no. 10, pp. 72–82. https://doi.org/10.18454/ACEN.2017.2.11
Rossini, P.M., Burke, D., Chen, R., et al., Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee, Clin. Neurophysiol., 2015; 126, pp. 1071–1107. https://doi.org/10.1016/j.clinph.2015.02.001
Di Lazzaro, V. and Ziemann, U., The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex, Front. Neural Circ., 2013, vol. 7, p. 18. https://doi.org/10.3389/fncir.2013.00018
Vucic, S., Ziemann, U., Eisen, A., et al., Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights, J. Neurol. Neurosurg. Psychiatry, 2013, vol. 84, pp. 1161–1170. https://doi.org/10.1136/jnnp-2012-304019
Vucic, S. and Kiernan, M.C., Transcranial magnetic stimulation for the assessment of neurodegenerative disease, Neurotherapeutics, 2017, vol. 14, pp. 91–106. https://doi.org/10.1007/s13311-016-0487-6
Geevasinga, N., Menon, P., Özdinler, P.H., et al., Pathophysiological and diag nostic implications of cortical dysfunction in ALS, Nat. Rev. Neurol., 2016, vol. 12, pp. 651–661. https://doi.org/10.1038/nrneurol.2016.140
Bakulin, I.S., Chervyakov, A.V., Suponeva, N.A., et al., Motor cortex hyperexcitability, neuroplasticity and degeneration in amyotrophic lateral sclerosis, in Novel Aspects of Amyotrophic Lateral Sclerosis, Foyaca-Sibat, H., Ed., Rijeka: InTech, 2016, pp. 47–72.
Vucic, S., Cheah, B.C., and Kiernan, M.C., Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis, Exp. Neurol., 2009, vol. 220, pp. 177–182. https://doi.org/10.1016/j.expneurol.2009.08.017
Bae, J.S., Simon, N.G., Menon, P., et al., The puzzling case of hyperexcitability in amyotrophic lateral sclerosis, J. Clin. Neurol., 2013, vol. 9, pp. 65–74. https://doi.org/10.3988/jcn.2013.9.2.65
Do-Ha, D., Buskila, Y., and Ooi, L., Impairments in motor neurons, interneurons and astrocytes contribute to hyperexcitability in ALS: underlying mechanisms and paths to therapy, Mol. Neurobiol., 2018, vol. 55, pp. 1410–1418. https://doi.org/10.1007/s12035-017-0392-y
Turner, M.R. and Kiernan, M.C., Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotrophic Lateral Scler., 2012, vol. 13, pp. 245–250. https://doi.org/10.3109/17482968.2011.636050
Clark, R., Blizzard, C., and Dickson, T., Inhibitory dysfunction in amyotrophic lateral sclerosis: future therapeutic opportunities, Neurodegener. Dis. Manage., 2015, vol. 5, pp. 511–525. https://doi.org/10.2217/nmt.15.49
Menon, P., Kiernan, M.C., and Vucic, S., Cortical hyperexcitability precedes lower motor neuron dysfunction in ALS, Clin. Neurophysiol., 2015, vol. 126, pp. 803–809. https://doi.org/10.1016/j.clinph.2014.04.023
Vucic, S., Nicholson, G.A., and Kiernan, M.C., Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis, Brain, 2008, vol. 131, no. 6, pp. 1540–1550. https://doi.org/10.1093/brain/awn071
van Zundert, B., Izaurieta, P., Fritz, E., and Alvarez, F.J., Early pathogenesis in the adult-onset neurodegenerative disease amyotrophic lateral sclerosis, J. Cell Biochem., 2012, vol. 113, pp. 3301–3312. https://doi.org/10.1002/jcb.24234
Vucic, S., Cheah, B.C., Yiannikas, C., and Kiernan, M.C., Cortical excitability distinguishes ALS from mimic disorders, Clin. Neurophysiol., 2011, vol. 122, pp. 1860–1866. https://doi.org/10.1016/j.clinph.2010.12.062
Attarian, S., Azulay, J.P., Lardillier, D., et al., Transcranial magnetic stimulation in lower motor neuron diseases, Clin. Neurophysiol., 2005, vol. 116, pp. 35–42.
Vucic, S. and Kiernan, M.C., Abnormalities in cortical and peripheral excitability in flail arm variant amyotrophic lateral sclerosis, J. Neurol. Neurosurg. Psychiatry, 2007, vol. 78, pp. 849–852.
Menon, P., Geevasinga, N., Yiannikas, C., et al., Cortical contributions to the flail leg syndrome: pathophysiological insights, Amyotrophic Lateral Scler. Frontotemporal Degener., 2016, vol. 17, pp. 389–396. https://doi.org/10.3109/21678421.2016.1145232
Geevasinga, N., Menon, P., Yiannikas, C., et al., Diagnostic utility of cortical excitability studies in amyotrophic lateral sclerosis, Eur. J. Neurol., 2014, vol. 21, pp. 1451–1457. https://doi.org/10.1111/ene.12422
Menon, P., Geevasinga, N., Yiannikas, C., et al., Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: a prospective study, Lancet Neurol., 2015, vol. 14, pp. 478–484. https://doi.org/10.1016/S1474-4422(15)00014-9
Poidasheva, A.G., Bakulin, I.S., Chernyavskii, A.Yu., et al., Motor cortex mapping with navigated transcranial magnetic stimulation and its clinical application, Med. Alfavit, 2017, no. 2, pp. 21–25.
de Carvalho, M., Miranda, P.C., Luís, M.L., and Ducla-Soares, E., Cortical muscle representation in amyotrophic lateral sclerosis patients: changes with disease evolution, Muscle Nerve, 1999, vol. 22, no. 12, pp. 1684–1692.
Chervyakov, A.V., Bakulin, I.S., Savitskaya, N.G., et al., Navigated transcranial magnetic stimulation in amyotrophic lateral sclerosis, Muscle Nerve, 2015, vol. 51, pp. 125–131. https://doi.org/10.1002/mus.24345
Menon, P., Kiernan, M.C., and Vucic, S., Cortical dysfunction underlies the development of the split-hand in amyotrophic lateral sclerosis, PLoS One, 2014, vol. 9, p. e87124. https://doi.org/10.1371/journal.pone.0087124
Devine, M.S., Pannek, K., Coulthard, A., et al., Exposing asymmetric gray matter vulnerability in amyotrophic lateral sclerosis, Neuroimage Clin., 2015, vol. 7, pp. 782–787. https://doi.org/10.1016/j.nicl.2015.03.006
Ravits, J., Paul, P., and Jorg, C., Focality of upper and lower motor neuron degeneration at the clinical onset of ALS, Neurology, 2007, vol. 68, pp. 1571–1575.
Menon, P., Geevasinga, N., van den Bos, M., et al., Cortical hyperexcitability and disease spread in amyotrophic lateral sclerosis, Eur. J. Neurol., 2017, vol. 24, pp. 816–824. https://doi.org/10.1111/ene.13295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare there is no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Rights and permissions
About this article
Cite this article
Bakulin, I.S., Poydasheva, A.G., Chernyavsky, A.Y. et al. Methods of Detecting Lesions of Upper Motor Neuron in Amyotrophic Lateral Sclerosis using Transcranial Magnetic Stimulation. Hum Physiol 45, 842–850 (2019). https://doi.org/10.1134/S0362119719080036
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119719080036