Abstract
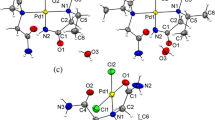
The new compound [Pd(NH2CH2CH2OH)4][Pd6(NH2CH2CH2S)8]Cl6 · 5H2O (I) is synthesized and its crystal structure is determined. The crystals are monoclinic, a = 25.625(6) Å, b = 9.633(5) Å, c = 24.847(7) Å, β = 91.47(2)°, Z = 4, and space group C2/c. The structural units of crystals I are the centrosymmetric hexanuclear [Pd6(NH2CH2CH2S)8]4+ cations, the mononuclear [Pd(NH2CH2CH2OH)4]2+ cations with C 2 symmetry, the Cl− anions, and crystallization water molecules. In the hexanuclear cation, the interaction between the Pd atoms occurs through the S atoms of the mercaptoethylaminate ligands. The Pd(2) and Pd(3) atoms and the ligands form two metallochelate fragments in which the N and S atoms are located in cis positions. The average lengths of the Pd-S and Pd-N bonds are equal to 2.274(1) and 2.074(6) Å, respectively. The metallochelate fragments are joined to each other and to their centrosymmetric analogues through the Pd(1) atom, which coordinates four S atoms [the average Pd-Sav bond length is 2.332(1) Å]. In the mononuclear cation, the Pd(4) atom coordinates four N atoms of the monoethylaminate ligands [the Pd-N bond lengths are 2.045(6) and 2.056(6) Å]. The shortest Pd⋯Pd distance is equal to 3.207(1) Å. The bonding in the structure is provided by numerous hydrogen bonds with the participation of all the H2O molecules, NH2 groups, and Cl− anions.
Similar content being viewed by others
References
D. C. Jicha and D. H. Bush, Inorg. Chim. Acta 1(4), 177 (1962).
I. A. Efimenko, Kh. I. Gasanov, N. A. Ivanova, et al., Koord. Khim. 26(2), 117 (2000).
I. A. Zakharova, in Research in Inorganic Chemistry and Chemical Technology (Nauka, Moscow, 1982), p. 171.
Yu. E. Gorbunova, Yu. N. Mikhailov, A. P. Kurbakova, and I. A. Efimenko, Koord. Khim. 19(4), 322 (1993).
I. A. Efimenko, Kh. I. Gasanov, Yu. E. Gorbunova, et al., Dokl. Akad. Nauk 326(4), 654 (1992).
Kh. I. Gasanov, S. S. Fatullaeva, D. I. Mirzai, and I. A. Efimenko, in Proceedings of the XVI Mendeleev Congress on General and Applied Chemistry, Moscow, 1998, p. 67.
S. Livingstone, Rhenium, Rhodium, Palladium, Osmium, Iridium, and Platinum (Pergamon, Oxford, 1975; Mir, Moscow, 1978).
S. I. Ginzburg, K. A. Gladyshevskaya, N. A. Ezerskaya, et al., Guide on Chemical Analysis of Platinum Metals and Gold (Nauka, Moscow, 1965).
V. A. Klimov, Basic Micromethods of Analyzing Organic Compounds (Khimiya, Moscow, 1967).
G. M. Sheldrick, SHELXS86: Program for the Solution of Crystal Structures (Univ. of Göttingen, Göttingen, 1986).
G. M. Sheldrick, SHELXL93: Program for the Refinement of Crystal Structures (Univ. of Göttingen, Göttingen, 1993).
Author information
Authors and Affiliations
Additional information
__________
Translated from Kristallografiya, Vol. 47, No. 4, 2002, pp. 660–666.
Original Russian Text Copyright © 2002 by Gasanov, Antsyshkina, Sadikov, Ivanova, Mirzai, Efimenko, Sergienko.
Rights and permissions
About this article
Cite this article
Gasanov, K.I., Antsyshkina, A.S., Sadikov, G.G. et al. Interaction of cystamine with palladium(II) monoethanolaminate complex: Crystal structure of [Pd(NH2CH2CH2OH)4][Pd6(NH2CH2CH2S)8]Cl6 · 5H2O. Crystallogr. Rep. 47, 603–609 (2002). https://doi.org/10.1134/1.1496058
Received:
Issue Date:
DOI: https://doi.org/10.1134/1.1496058