Abstract
Invasion of periodontal tissues by Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans can be associated with aggressive forms of periodontitis. Oleoresins from different copaifera species and their compounds display various pharmacological properties. The present study evaluates the antibacterial and antivirulence activity of oleoresins obtained from different copaifera species and of ten isolated compounds against two causative agents of periodontitis. The following assays were performed: determination of the minimum inhibitory concentration (MIC), determination of the minimum bactericidal concentration (MBC), and determination of the antibiofilm activity by inhibition of biofilm formation and biofilm eradication tests. The antivirulence activity was assessed by hemagglutination, P. gingivalis Arg-X and Lis-X cysteine protease inhibition assay, and A. actinomycetemcomitans leukotoxin inhibition assay. The MIC and MBC of the oleoresins and isolated compounds 1, 2, and 3 ranged from 1.59 to 50 μg/mL against P. gingivalis (ATCC 33277) and clinical isolates and from 6.25 to 400 μg/mL against A. actinomycetemcomitans (ATCC 43717) and clinical isolates. About the antibiofilm activity, the oleoresins and isolated compounds 1, 2, and 3 inhibited biofilm formation by at least 50% and eradicated pre-formed P. gingivalis and A. actinomycetemcomitans biofilms in the monospecies and multispecies modes. A promising activity concerning cysteine protease and leucotoxin inhibition was also evident. In addition, molecular docking analysis was performed. The investigated oleoresins and their compounds may play an important role in the search for novel sources of agents that can act against periodontal pathogens.
Similar content being viewed by others
Periodontitis is a polymicrobial infection originating from excessive pathogenic biofilm accumulation at the gingival margin, that leads to inflammation in tooth-supporting tissues (i.e., the periodontium)1. As periodontitis develops, plaque previously dominated by aerobic species transitions to plaque where strict and facultative anaerobic species such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans prevail2,3.
P. gingivalis is an important Gram-negative anaerobic bacteria and stands out as a key pathogen in chronic periodontitis: this species plays a disproportionately important role in depressing and deregulating local immune responses, which culminates in increased virulence of the entire community and causes periodontitis dysbiosis4. This bacterium is known to produce a repertoire of virulence factors that could penetrate the gingivae and destroy the tissue directly or indirectly by inducing inflammation5,6. The virulence factors of this bacterium include fimbriae, capsules, lipopolysaccharide (LPS), lipoteichoic acids, hemagglutinins, gingipains, outer membrane proteins, and outer membrane vesicles6,7,8.
A. actinomycetemcomitans is a Gram-negative bacterium, facultative anaerobe that is associated with the etiology of aggressive periodontitis. This bacterium is also associated with non-oral infections, such as endocarditis, and is a candidate bacterial trigger of anti-citrulline autoimmunity in rheumatoid arthritis9,10,11. Some virulence factors of leukotoxin (ltx) are related to evasion of host defense and destruction of the host’s tissues12.
The A. actinomycetemcomitans virulence potential varies among strains, and specific serotypes/clonal types of this bacterium predominate in individuals with aggressive forms of periodontal disease13. LtxA is a large pore-forming toxin that belongs to the family of bacterial proteins RTX (Repeats-in-toxin). LtxA expression varies widely in vitro although all A. actinomycetemcomitans strains have a complete ltxA operon. LtxA expression has not been fully characterized, but environmental and genetic factors regulate its expression14,15,16.
The main goal of non-surgical periodontal therapy is to control microbial periodontal infection by removing bacterial biofilm, calculus, and toxins from periodontally involved root surfaces17. Facilitating antibiotic diffusion into biofilms requires agents that can penetrate and destroy the components of the biofilm matrix and kill bacteria18.
Over the last decades, phytodrugs have assumed a prominent part as possible alternative therapy in dentistry19. Researchers have been interested in plants with antimicrobial properties and bactericidal potential. Plants belonging to the genus Copaifera L. (Fabaceae-Caesalpinioideae), popularly known as ‘copaiba’ in Brazil, are native to the tropical regions of Latin America and Western Africa. Copaifera oleoresin obtained from the trunk of these trees has become prominent in Brazilian Natural Medicine20.
Oleoresin is a product of the secondary metabolism of plants, and it defends the plant against animals, fungi, and bacteria. Oleoresins contain mainly diterpenes, including kaurenoic acid, kaurenol, copalic acid, agathic acid, hardwickiic acid, polyalthic acid, and the sesquiterpenes β-caryophyllene, karyophylene oxide, α-copaene, α-humulene, and β-bisabolol, among other compounds21. In this sense, our research group has been devoted to investigating Copaifera spp.2,22,23,24,25,26,27,28,29,30,31.
Several studies have highlighted the promising potential of Copaifera spp. against bacteria that cause endodontic infections and caries2,24,25,26,29. Therefore, here we aimed to evaluate the antibacterial and antivirulence activity of the oleoresins obtained from C. paupera, C. pubiflora, and C. reticulata and isolated compounds against P. gingivalis and A. actinomycetemcomitans, which are important pathogens in the development of periodontitis, as well investigation of the interactions between the compounds and bacteria through molecular docking analysis.
Material and methods
Plant material and isolated compounds
Authentic Copaifera oleoresins were collected in different Brazilian States between 2011 and 2014, and voucher specimens were deposited in the Herbarium of the Brazilian Agricultural Research Corporation (Embrapa Eastern Amazon) and identified as Copaifera paupera (Herzog) Dwyer (Xapuri, state of Acre, NID 10/2014), C. pubiflora Benth (Mucajaí, state of Roraima, NID 15/2014), and C. reticulata Ducke (Brasil Novo, state of Pará, NID 03/2013) by Silvane Tavares Rodrigues30. The isolated compounds (Fig. 1) polyalthic acid (1), kaurenoic acid (2), hardwickiic acid (3), ent-copalic acid (4) and its isomer [(13E)-ent-labda-7,13-dien-15-oic acid] (5), ent-agathic acid (6), ent-agathic acid 15-methyl ester (7), ent-agatholic acid 15-methyl ester (8), ent-agatholic acid (9), and junenol (10) were isolated from the oleoresins obtained from Copaifera spp. and identified by our research group as described previously22,23,30,32,33,34.
Chemical structures of the tested compounds: polyalthic acid (1), kaurenoic acid (2), hardwickiic acid (3), ent-Copalic acid (4) and its isomer [(13E)-ent-labda-7,13-dien-15-oic acid; 5], ent-agathic acid (6), ent-agathic acid 15-methyl ester (7), ent-agatholic acid 15-methyl ester (8), ent-agatholic acid (9), and junenol (10).
Bacterial strains and antimicrobial assays
The periodontal disease-causing bacteria that were used in this study included standard strains and clinical isolates, namely P. gingivalis ATCC 33277 and clinical isolate and A. actinomycetemcomitans ATCC 43717 and clinical isolate. The standard strains were obtained from the American Type Culture Collection (ATCC). These bacteria were kept in the UNIFRAN culture collection under cryopreservation at − 80 °C.
The research participants (fifteen subjects) were selected from patients that came to the Dental School, University of Franca, UNIFRAN, Franca, SP, Brazil for periodontic treatment. After the periodontal pockets were evaluated, collections were carried out for a maximum of 5 min, to ensure that the sample was stable. One sample was collected per patient, and the most productive pouch was chosen (> 5 mm deep).
The inclusion criteria were as follows: patients aged over 18 years, of both genders, who had indication for periodontal treatment with over 5-mm deep gingival pockets in at least one of the four facets of the analyzed tooth, and who were examined a dental professional. The patient needed to have at least four teeth.
The exclusion criteria were patients with a history of systemic disease such as rheumatic fever, coronary heart disease, and respiratory diseases; patients that had received systemic or local antibiotic therapy in the six previous months; patients that had used antibiotics or anticoagulants; and patients that had undergone periodontal treatment or dental prophylaxis in the previous six months.
The clinical isolates were collected from patients after approval of the Research Ethics Committee of the University of Franca—CAAE 41530915.9.0000.5495, in accordance with international protection guidelines and the Helsinki Declaration. All the patients signed an informed consent for their participation in this research.
The P. gingivalis and A. actinomycetemcomitans species were isolated according to the methodology of Esfahani et al.35. The species were obtained from patients with chronic and aggressive periodontitis. Ten strains were selected from the total number of isolated and identified bacteria, namely five P. gingivalis strains and five A. actinomycetemcomitans strains. The Polymerase Chain Reaction (PCR) technique was used to detect the P. gingivalis 16SrDNA and A. actinomycetemcomitans 16SrDNA genes. DNA was extracted from the strains by employing the Pure Link Microbiome DNA purification kit (Invitrogen, Carlsbad, California, USA); the manufacturer's instructions were followed. The PCR technique was conducted as described by Wu et al.36.
The Minimum Inhibitory Concentration (MIC; the lowest concentration of the test compound that can inhibit microorganism growth) and the Minimum Bactericidal Concentration (MBC; the lowest concentration of the test compound at which no bacterial growth occurs) were determined as described by Abrão et al.29.
The antibiofilm activity was investigated by using two distinct methodologies, in two modes, monospecies and multispecies biofilm. In the first methodology, the Minimum Inhibitory Concentration of Biofilm (MICB50) of the most promising metabolites against the bacteria evaluated in this study was determined according to the methodology described by Abrão et al.29, on the basis of the minimum concentration of antimicrobial agent that inhibited biofilm formation by at least 50%. In the second methodology, the Minimum Concentration of Biofilm Eradication (MCBE), defined as the lowest concentration that reduced the number of biofilm cells by at least 99.9%, was determined as described by Souza et al.31.
Antivirulence assays
The oleoresins and isolated compounds were used to inhibit virulence factors. The antivirulence assays included assessment of the P. gingivalis and A. actinomycetemcomitans hemagglutination activity, P. gingivalis gingipaine inhibition, and A. actinomycetemcomitans leukotoxin inhibition.
Hemagglutination assay
To perform the hemagglutination assay, which allowed fimbriae to be detected, the methodology of Kikuchi et al.37 was applied. P. gingivalis and A. actinomycetemcomitans colonies that had been grown for five days and 24 h, respectively, were suspended in PBS buffer (Sigma) pH 7.4 and adjusted to an absorbance of 2.0 at 660 nm. The oleoresins and isolated compounds were tested at subinhibitory concentrations (½ MIC). To this end, 150 μL of an oleoresin or isolated compound was mixed with 150 μL of the bacterial suspension, which was followed by incubation at appropriate temperature and atmosphere for each strain (anaerobic chamber at 36 °C for P. gingivalis strains and CO2 chamber at 36 °C for A. actinomycetemcomitans strains) in sterile Eppendorf tubes. After incubation, the bacteria were centrifuged at 6000× g for 5 min, resuspended with 500 μL of PBS buffer (Sigma) pH 7.4, and diluted to 1/32 in 96-well plates. A 50-μL aliquot of each dilution was homogenized with 50 μL of a 2% fresh red blood cell suspension in PBS (Sigma) pH 7.4 and incubated in appropriate atmosphere for 3 h. Hemagglutination was visually assessed after incubation.
Gingipain inhibition assay
The activity of lysine (Lys-X encoded by the Kgp gene) and arginine (Arg-X, encoded by the RgpA and RgpB genes) gingipaines was determined by using N-(p-tosyl)-Gly-Pro-Lys 4-nitroanilide acetate synthetic substrate salt (Sigma-Aldrich) and Nα-benzoyl-L-arginine-7-amido-4-methyl-coumarin hydrochloride (Sigma-Aldrich), respectively, according to the methodology described by Fujise et al.38 and Chen et al.39, respectively.
The P. gingivalis strains were grown in Brucella broth in anaerobic chamber at 36 °C for 72 h. Then, the bacteria were centrifuged at 6000× g for 5 min and resuspended with PBS (Sigma) pH 7.4 buffer containing 1 mM L-cysteine, to adjust the absorbance at 660 nm to 0.26, and the resulting suspensions were added to the 96-well microplate. The oleoresins and isolated compounds were tested against the P. gingivalis strains at subinhibitory concentrations (½ MIC).
The Arg-X activity was assayed in 100 μL of PBS containing 1 mM L-cysteine, 100 μL of substrate, and 5 μL of bacterial suspension. The Lis-X activity was tested in 100 μL of PBS containing 1 mM L-cysteine, 100 μL of substrate, and 50 μL of bacterial suspension. The 7-starch-4-methylcoumarin that was released due to cleavage of the substrates was measured on a fluorimeter (Ascent FL, Thermoscientific, Waltham, MA, USA) upon excitation at 365 nm and emission at 460 nm. The proteolytic activity was compared to the untreated control40.
Leukotoxin inhibition assay
This test was carried out by following the methodology described by de Lima et al.41. Mononuclear human leukocytes (LMNS) were isolated from an enriched fraction of venous blood leukocytes by using Histopaque 1077 (Sigma-Aldrich); the manufacturer's instructions were followed. The LMNS-containing fraction was collected, and the cells were washed three times with PBS (1000 rpm for 5 min). The cell pellet was resuspended to a concentration of 5 × 106 cells/mL in RPMI culture medium containing L-glutamine, 10% fetal bovine serum, and penicillin–streptomycin (Sigma-Aldrich). The leukocyte fraction was mixed with an oleoresin or isolated compound at a ratio of 106 cells to 100 μL of oleoresin or isolated compound in sterile tubes. Triton X100 at 0.1% was the positive control. The A. actinomycetemcomitans strains were cultivated in TSA broth in a CO2 chamber at 36 °C for 24 h. After incubation, the strains were centrifuged at 9000 rpm and 4 °C for 15 min, and the pellet was sonicated (16 kHz at 200 W) to obtain the proteins. After sonication, the supernatant was separated into a sterile tube, and the amount of protein was dosed with the Bicinchoninic Acid Kit for Protein Determination, Sigma-Aldrich). An amount of 500 μg/mL protein was added to sterile tubes containing leukocyte and an oleoresin or isolated compound and incubated in the CO2 chamber at 37 °C for 1 h. After incubation, the previously prepared trypan blue solution was added to a concentration of 1.6 mg/mL and kept in the CO2 chamber for seven minutes. After that, the dead cells were counted with the aid of the Neubauer chamber, and the results were graphically expressed.
Molecular docking
The interaction of the isolated compounds polyalthic acid, kaurenoic acid, and hardwickiic acid with the active sites of the enzymes A. Arg-X (PDB ID 1CVR) and B. Lis-X gingipain (PDB ID 6I9A) from P. gingivalis was evaluated my molecular docking. The structure of Lis-X was downloaded in .pdb format and imported into Discovery Studio. The structure was prepared using pH 7.4 for assigning the protonation states of polar hydrogens. The prepared protein was then imported into GOLD, and the active site was defined based on the position of the co-crystallized ligand and on the annotation of active site 5 as found on the .pdb file (xyz coordinated 3.946969 -15.587595 8.143534, 20 Å). The structure of Arg-X handled similarly and prepared with GOLD42,43. The active site 1 was used, based on the position of the co-crystallized ligand and assignment on the .pdb file (xyz coordinates 57.619488 23.562437 54.075112, 10 Å). 3D structures of the ligands were downloaded from PubChem and further charge states were generated with Discovery Studio, covering pHs 6.5 to 8.5. Molecular docking was run on GOLD42,43 (genetic algorithm approach set to most accurate) and the GoldScore fitness function was used for ranking the poses. 3D Ligand interaction diagrams (LIDs) were prepared with Maestro Release 2020-2, Schrödinger, LLC, New York, NY, 2020. Final illustrations were prepared with the open-source distribution of Pymol, version 2.4.0b0.
Statistical analysis
Statistical analysis of the data obtained from the gingipain inhibition assay and leukotoxin inhibition assay was accomplished by One Way ANOVA analysis and Tukey's test with the aid of the software GraphPad Prism version 5.00.
Results and discussion
MIC and MBC
We used strains that were isolated from patients diagnosed with chronic and aggressive periodontitis. More specifically, we detected five P. gingivalis strains and five A. actinomycetemcomitans strains and named them PG01 to PG05 and AA01 to AA05, respectively. PCR with the 16srDNA gene helped to confirm the strains. We evaluated the C. paupera, C. pubiflora, and C. reticulata oleoresins and isolated compounds against the P. gingivalis and A. actinomycetemcomitans strains isolated from patients with periodontitis and against standard strains. Table 1 summarizes the results of these assays.
Regarding the tested P. gingivalis strains, the MIC and MBC values of the C. reticulata oleoresin ranged from 3.12 to 12.5 μg/mL. The MBC results revealed a bactericidal effect against all the strains. The exception was P. gingivalis ATCC 33277, against which the C. reticulata oleoresin exerted a bacteriostatic action. The C. paupera oleoresin provided MIC and MBC values between 3.12 and 50 μg/mL and had a bactericidal effect against all the strains. The C. pubiflora oleoresin exhibited MIC and MBC values between 3.12 and 50 μg/mL and was bacteriostatic only against P. gingivalis (ATCC 33277). Polyalthic acid (1) afforded MIC and MBC values between 3.12 and 12.5 μg/mL and displayed a bactericidal effect against all the strains. Kaurenoic acid (2) provided MIC and MBC values of 6.25 μg/mL. Hardwickiic acid (3) exhibited MIC and MBC results ranging from 1.59 to 25 μg/mL and exhibited bactericidal activity against PG03, PG04, PG05, and P. gingivalis (ATCC 33277).
Concerning the assayed A. actinomycetemcomitans strains, the C. reticulata oleoresin afforded MIC and MBC results between 25 and 100 μg/mL and exerted a bacteriostatic effect only against A. actinomycetemcomitans (ATCC 43717). The MIC and MBC values of the C. paupera oleoresin varied from 12.5 to 100 μg/mL, and this oleoresin had a bacteriostatic effect against AA04 and A. actinomycetemcomitans (ATCC 43717). The C. pubiflora oleoresin exhibited MIC and MBC results between 6.25 and 50 μg/mL and displayed a bactericidal effect against AA01, AA02, and AA04. This same oleoresin exhibited a bacteriostatic effect against AA03, AA05, and A. actinomycetemcomitans (ATCC 43717). Isolated compound 1 afforded MIC and MBC results between 6.25 and 50 μg/mL and had a bacteriostatic effect against AA03 and AA05. Isolated compound 2 provided MIC and MBC values between 25 and 400 μg/mL and exerted a bactericidal effect against all the A. actinomycetemcomitans strains except AA02. Isolated compound 3 exhibited MIC and MBC results between 6.25 and 25 μg/mL and presented a bacteriostatic effect against AA02 and AA04.
As for isolated compounds 4–10, they afforded MIC values higher than 10 μg/mL against all the tested P. gingivalis and A. actinomycetemcomitans strains. According to Ríos and Récio44 and Gibbons45, only crude plant extracts with MIC values lower than 100 μg/mL and isolated substances with MIC values lower than 10 μg/mL are considered as promising sources of antibacterial agents.
Few articles have addressed the antibacterial activity of the oleoresins and isolated compounds investigated in this study. Tincusi et al.46 evaluated the antibacterial activity of terpenoids isolated from the C. paupera oleoresin against Gram-positive strains. The authors highlighted the results they achieved with copalic acid, polyalthic acid, and kaurenoic acid (MIC lower than 10 μg/mL) against Gram-positive strains and the antibacterial potential of the C. paupera oleoresin, used in traditional Peruvian medicine.
In a recent work, Furtado et al.30 evaluated the cytotoxic and genotoxic activity of oleoresins obtained from six Copaifera species, including C. reticulata, C. paupera, and C. pubiflora. The authors employed the clonogenic efficiency assay to determine the cytotoxicity of the oleoresins extracted from the different species. On the basis of the cytotoxicity results, they selected C. paupera and C. pubiflora for the genotoxicity assay. Testing with V79 cells (Chinese hamster fibroblasts) revealed that all the oleoresins are cytotoxic: IC50 values range from 9.8 to 99.2 μg/mL, but none of the oleoresins is cytotoxic at 2000 mg/kg. Compared to the negative control, V79 cell cultures treated with the Copaifera oleoresins do not have significantly different frequency of micronuclei, demonstrating the absence of genotoxic effect. Here, the C. reticulata, C. paupera, and C. pubiflora oleoresins showed cytotoxicity: their IC50 values were 60.8, 40.6, and 54.0 μg/mL, respectively. Comparison of these IC50 values with the MIC results (from 3.12 to 12.5 μg/mL for C. reticulata, from 3.12 to 50 μg/mL for C. paupera, and from 3.12 to 25 μg/mL for C. pubiflora against the evaluated P. gingivalis strains) showed that microorganism inhibition was due to the oleoresin antibacterial activity and not to the cytotoxic action. With respect to the tested A. actinomycetemcomitans strains, the MIC values of the oleoresin were higher than the IC50 values found by Furtado et al.30, but we also attributed microorganism inhibition to the antibacterial activity and not to the cytotoxicity of the oleoresins.
According to Bakri and Douglas47, for new therapeutic agents to translate into effective in vivo therapies for periodontitis, they must be active against biofilms rather than just planktonic cells. Alves et al.48 stated that antibiofilm compounds act in distinct ways: they can have a preventive effect through inhibition of biofilm formation or a therapeutic effect by acting on already established biofilms. Therefore, we decided to evaluate the two possible antibiofilm activities by conducting biofilm inhibition and eradication assays.
Antibiofilm assays
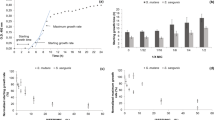
To carry out the biofilm inhibition and eradication tests on the P. gingivalis and A. actinomycetemcomitans strains, first we had to verify the ability of the analyzed strains to grow in the sessile mode. All the strains formed monospecies and multispecies biofilms. In the antibiofilm activity assay, we evaluated concentrations from 0.78 to 1600 µg/mL (Fig. 2A–F). To determine the anti-biofilm activity of the oleoresins and isolated compounds, we used two techniques: optical density reading (which evaluates the presence of biofilm mass) at 570 nm and microorganism count expressed in log10 and colony forming units per milliliter (CFU/mL).
Concerning the inhibitory activity of the oleoresins against the monospecies biofilms, the C. paupera oleoresin exhibited IC50 and MICB50 of 95.37 and 25.0 µg/mL, respectively, against PG03 (Fig. 2A). The C. pubiflora oleoresin provided IC50 and MICB50 of 186.1 and 12.5 µg/mL, respectively, against AA04 (Fig. 2C). In the case of the multispecies mode, the C. pubiflora oleoresin at 800 and 1600 µg/mL displayed high antibacterial activity, with IC50 of 60.17 µg/mL and MICB50 of 400 µg/mL against a combination of AA04 and PG03 (Fig. 2E).
According to Fux et al.49, the drug concentration that is required to kill bacteria in the sessile mode may be 10 to 1000 times higher than the concentration that is necessary to kill bacteria in the planktonic mode. Here, the MICB50 values of the evaluated oleoresins and isolated compounds against the monospecies mode ranged from 12.5 to 100 μg/mL (Fig. 2A–D). For the tested P. gingivalis strains, the MICB50 values were 8 to 251 times higher than the MIC values, corroborating the data established by Fux et al.49. With respect to the assayed A. actinomycetemcomitans strains, the MICB50 values were 2 to 16 times higher than the MIC values and are considered promising for anti-biofilm activity.
Souza et al.31 evaluated the anti-biofilm activity of ent-copalic acid against Actinomyces naeslundii and Peptoestreptococcus anaerobius, which cause endondontic infections. ent-Copalic acid at 500 and 2000 μg/mL inhibits biofilm formation by 50% for A. naeslundii and P. anaerobius. Herein, isolated compounds 1, 2, and 3 presented MICB50 of 50, 100, and 200 μg/mL against the monospecies and multispecies biofilms (Fig. 2B,D,F). Therefore, our results were superior to the results of Souza et al.31.
Abrão et al.29 assessed the anti-biofilm activity of C. duckei and its isolated compound polyalthic acid against P. gingivalis (ATCC 33277) and found MICB50 of 200 μg/mL for the oleoresin and 6.25 μg/mL for polyalthic acid. In our studies, the C. pubiflora and C. paupera oleoresins exhibited MICB50 of 12.5 and 25 μg/mL against the A. actinomycetemcomitans and P. gingivalis clinical isolates, respectively (Fig. 2A,C). Regarding isolated compound 1, which exhibited MICB50 of 50 μg/mL against PG03 (Fig. 2B), which was lower than the result found in the studies by Abrão et al.29. These results confirmed the potential antibiofilm action of the Copaifera species oleoresins and their isolated compounds and reaffirmed the selectivity toward more resistant strains in the bacterial biofilm of patients with periodontal pouch50,51.
Graphs in Fig. 3 illustrate the biofilm eradication activity of the investigated oleoresins and isolated compounds against the tested bacteria in the monospecies and multispecies modes. In the monospecies mode, isolated compound 3 provided the best activity against PG03 (IC50 of 55.79 µg/mL) (Fig. 3B), whereas the C. paupera oleoresin exhibited the best result against A. actinomycetemcomitans (ATCC 43717) (IC50 of 58.66 µg/mL) (Fig. 3C). With respect to the multispecies mode, isolated compound 3 eradicated the combination of AA03 and PG03 with IC50 of 278.7 µg/mL (Fig. 3E).
Souza et al.31 evaluated the biofilm eradication activity of ent-copalic acid against A. naeslundii and P. anaerobius biofilms. These authors calculated the Minimum Inhibitory Concentration of Biofilm Eradication, which is defined as the concentration of the substance that is able to reduce biofilm cells by 99.9%. The ent-copalic acid at 1000 and 62.5 μg/mL eradicates A. naeslundii biofilm cells and P. anaerobius biofilm cells, respectively. We determined the biofilm eradication activity on the basis of IC50, which is defined as the concentration of an inhibitor that results in half the inhibition of a response as compared to the control group52. In the case of the P. gingivalis clinical isolates, the IC50 of the evaluated oleoresins and isolated compounds ranged from 94.02 to 282.3 μg/mL and from 55.79 to 419.6 μg/mL, respectively (Fig. 3A,B). For A. actinomycetemcomitans, the oleoresins and isolated compounds exhibited IC50 from 58.66 to 189.4 μg/mL and from 95.68 to 265.6 μg/mL, respectively (Fig. 3C,D). These results were more promising than the values reported by Souza et al.31.
In their natural environments, most biofilms probably consist of a consortium of species that influence each other synergistically or antagonistically. However, there is little knowledge of their structure, characteristics (including community dynamics), and response to antimicrobial agents53.While monospecies biofilms have been extensively studied, little is known about multispecies biofilms and their interactions53. Interactions between microorganisms are complex and play an important role in the pathogenesis of infections. These interactions may range from fierce competition for nutrients and niches to highly evolved cooperative mechanisms between different species that support their mutual growth54.
Hemagglutination assay
Herein, we evaluated the oleoresins and isolated compounds at sub-inhibitory concentrations. Concerning the A. actinomycetemcomitans strains, the oleoresins and isolated compounds inhibited the hemagglutination activity at all the assayed dilutions. Controls were performed by assessing the ability of all the strains to hemagglutinate blood suspension. All the P. gingivalis strains were capable of hemagglutination, and the oleoresins and isolated compounds inhibited their hemagglutination activity at all the tested dilutions. In P. gingivalis, the hemagglutination capacity is associated with the process of adhesion to gum cells and later lysis of red blood cells for iron capture55,56,57,58.
A. actinomycetemcomitans exhibits many virulence factors (fimbriae, hemagglutinin, capsule, lipopolysaccharide, outer membrane vesicles, and enzymatic activities) that can disrupt host defense mechanisms and initiate tissue destruction; however, no specific invasion mechanism has been identified59. For Nakagawa et al.60, the A. actinomycetemcomitans cellular invasion rate is low as compared to P. gingivalis. In the studies by Meyer et al.61, invasion of human cells by A. actinomycetemcomitans was limited to some bacterial strains, approximately 25% of invasive A. actinomycetemcomitans. Löhr et al.62 evaluated the ability of polyphenols obtained from Myrothamnus flabellifolia to inhibit hemagglutination in the case of P. gingivalis, and they found that the polyphenols at 1000, 500, 100, 50, and 1 μg/mL inhibit this activity at all the tested dilutions (1 to 1:64). In the present study, we examined subinhibitory concentrations of the oleoresins and isolated compounds, from 0.79 to 3.12 μg/mL, and verified the promising potential of the C. paupera, C. pubiflora, and C. reticulata oleoresins and their isolated compounds 1, 2, and 3 to inhibit the P. gingivalis and A. actinomycetemcomitans hemagglutination activity.
Gingipain inhibition assay
We evaluated the ability of the C. paupera, C. pubiflora, and C. reticulata oleoresins and isolated compounds 1, 2, and 3 to inhibit the gingipain activity of the P. gingivalis strains PG01 and PG03. The tested concentrations were sub-inhibitory, and the results are shown in Fig. 4A,B.
Inhibition of the Arg-X and Lys-X enzymatic activity (%) by the C. reticulata, C. paupera, and C. pubiflora oleoresins (A) and the isolated compounds kaurenoic acid (B), polyalthic acid, and hardwickiic acid (A). Values are expressed as mean and standard deviation in triplicate. Double asterisks (P < 0.01) and triple asterisks (P < 0.001) indicate significantly different values versus control values. Data were analyzed using GraphPad Prism software version 5.00 (available https://www.graphpad.com/scientific-software/prism/).
The C. reticulata, C. paupera, and C. pubiflora oleoresins inhibited the Arg-X enzymatic activity by 78.81 ± 4.02%, 63.71 ± 1.62%, and 51.47 ± 7.86%, respectively, and the Lis-X enzymatic activity by 72.09 ± 3.59%, 54.24 ± 8.11%, and 62.66 ± 6.13%, respectively (Fig. 4A). Isolated compounds 1, 2, and 3 inhibited the Arg-X enzymatic activity by 66.11 ± 3.28%, 80.04 ± 2.69%, and 54.78 ± 8.11%, respectively, and the Lis-X enzymatic activity by 61.94 ± 4.38%, 74.66 ± 4.21%, and 51.10 ± 3.29%, respectively (Fig. 4A,B). The proteases produced by P. gingivalis play a crucial role in this assacarolytic bacterium: this microorganism does not break down carbohydrates to produce energy, so it needs to degrade host proteins and amino acids63. P. gingivalis proteases are known as gingipaines and include lysine-specific gingipaines, encoded by the Kpg gene, and arginine-specific gingipaines, encoded by the RgpA and RgpB genes64.
Löhr et al.62 investigated the ability of Myrothamnus flabellifolia Welw polyphenols to inhibit the Arg-X and Lis-X enzymatic activity. After contact with 1 μg/mL polyphenols for 1 min, inhibition is 50%. Prolonged incubation does not increase the anti-protease effects. Polyphenols at 50 and 100 μg/mL inhibit Arg-gingipain by 70–80% and by about 80%, respectively. As for Lys-gingipain, inhibition is only 50%. The authors indicated that M. flabellifolia polyphenol is a potent inhibitor of Arg-X activity.
Here, we evaluated the antivirulence activity of the oleoresins obtained from the Copaifera species and isolated compounds by the Arg-X and Lis-X enzyme inhibition assay. The oleoresins and isolated compounds inhibited at least 50% of the Arg-X and Lys-X enzymatic activity (Fig. 4).
The structures of Arg-X and Lis-X gingipaines are described as resembling a tooth65,66, with a crown encompassing the N-terminal subdomain (NSD) and C-terminal subdomain (CSD), see Supplementary Fig. 1. The catalytic site is part of the CSD. The “root” is composed of a IgSF. The hydrolase activities (Cys-proteinase, EC 3.4.22.47) of Arg-X and Lis-X gingipaines are related to different sets of catalytic residues. For Arg-X, H444 and C477 form the the catalytic dyad, while for the Lis-X enzyme the catalytic triad is composed by H444, C477 and D388, as shown in recent studies66.
To obtain further information on the mechanism of inhibition of Arg-X and Lis-X gingipain, the GOLD molecular docking suite was used42,43. The isolated compounds polyalthic acid, kaurenoic acid, and hardwickiic acid were docked into the active sites of the enzymes Arg-X (PDB ID 1CVR) and Lis-X gingipain (PDB ID 6I9A) from P. gingivalis (Fig. 5). The results obtained here, in combination with the ligand interaction diagrams, demonstrate that all three free compounds can interact with the active sites of both enzymes. Interestingly, the compounds endowed with the furan-containing flexible “arm” (polyalthic acid and hardwickiic acid) could be expected to bind more tightly with the active site binding pocket. This trend is observed in the GoldScore Fitness scores. For Lys-X, polyalthic acid and hardwickiic generated posed with better interaction with the active site of the enzyme than kaurenoic acid (See Table 2). For Arg-X, on the other hand, the only compound that was predicted to bind tightly with the active site was polyalthic acid (see Table 2).
The interaction of the isolated compounds kaurenoic acid, polyalthic acid, and hardwickiic acid with the active sites of the enzymes (A). Arg-X (PDB ID 1CVR) and (B). Lis-X gingipain (PDB ID 6I9A) from P. gingivalis was evaluated my molecular docking using the GOLD42,43. The active sites of each enzyme are also shown. For Arg-X, H444 and C477 form the the catalytic dyad, while for the Lis-X enzyme the catalytic triad is composed by H444, C477 and D388, as shown in recent studies4. Ligand interaction diagrams (LIDs) shown in details the intermolecular interactions in each case. LIDs were prepared with Maestro Release 2020-2, Schrödinger, LLC, New York, NY, 2020.
The molecular docking analysis indicates that, although some of the compounds are predicted to have the potential to bind to the active sites of both Arg-X and Lys-X, gingipaines, this factor alone does not account to for inhibitory potencies observed experimentally in vitro. It suggests that other forms of inhibition are taking place experimentally. Allosteric inhibition, for example, is not uncommon for proteases67.
Leukotoxin inhibition assay
We exposed different concentrations of the C. paupera, C. pubiflora, and C. reticulata oleoresins and isolated compounds 1, 2, and 3 (MIC, ½ MIC, and 2 × MIC) to 106 cells/mL of LMNS and 500 μg/mL of A. actinomycetemcomitans proteins. The results in Fig. 6A–F showed that the oleoresins and the isolated compounds inhibited A. actinomycetemcomitans leucotoxins: they reduced the leukocyte mortality percentage to less than 50%.
Mortality percentage of human mononuclear cells after exposure to the oleoresins (A,C,E) and the isolated compounds kaurenoic acid (B), polyalthic acid (F), and hardwickiic acid (D) and the control (Triton-X). Values are expressed as mean and standard deviation in triplicate. Triple asterisks (P < 0.001) indicate significantly different values versus control values. Data were analyzed using GraphPad Prism software version 5.00 (available https://www.graphpad.com/scientific-software/prism/).
The ltxA gene encodes leukotoxin; the ltxB and ltxD genes encode proteins required for toxin secretion; and the ltxC gene encodes acyl transferase production, which underlies toxin transformation from protoxin to the active form51,68. The presence of leukotoxin has been associated with the A. actinomycetemcomitans ability to escape the main line of defense in the periodontal pocket and contributes to the pathogenesis of periodontal disease51,69. Leukotoxic activity is determined by a cytolytic action that kills human polymorphonuclear leukocytes, T lymphocytes, and macrophages. In contrast, epithelial and endothelial cells, fibroblasts, and platelets are resistant to this action51,69,70.
In the present study, we assessed the A. actinomycetemcomitans leukotoxin inhibition by counting the number of viable cells by Trypan Blue staining. This methodology is based on the observation that viable cells are impermeable to the dye, whereas nonviable cells are permeable to Trypan Blue, which enters pores in the cell membrane71. Here, exposure of the A. actinomycetemcomitans strains to the C. paupera, C. pubiflora, and C. reticulata reduced leukocyte mortality to between 15.93 and 31.19% (Fig. 6A,C,E). Isolated compounds 1, 2, and 3 diminished leukocyte mortality to between 11.44 and 39.83% (Fig. 6B,D,F). There are no literature data on the inhibition of A. actinomycetemcomitans leukotoxins by compounds isolated from natural products, so we could not compare the efficiency of the oleoresins and isolated compounds evaluated in the present study with literature results. Compared to the control group, the oleoresins and isolated compounds abated the effect of leukotoxins and decreased leukocyte mortality to below 50%.
Conclusion
The C. paupera, C. pubiflora, and C. reticulata oleoresins and the isolated compounds polyalthic acid, kaurenoic acid, and hardwickiic acid have promising antibacterial activity and monospecies and multispecies antibiofilm activity against major pathogens that cause periodontitis, namely A. actinomycetemcomitans and P. gingivalis. Promising antivirulence activity was evident in the hemagglutination assays, P. gingivalis cysteine protease inhibition (Arg-X and Lys-X) assays, and A. actinomycetemcomitans leukotoxin inhibition assays. The mechanism of inhibition of Arg-X and Lys-X by the isolated compounds was further studies by molecular docking. Direct binding of the small molecules to the active site of the gingipaines does not completely explain the potent inhibitory activities of the compounds observed experimentally (> 74% for kaurenoic acid, for example). Therefore, allosteric inhibition is proposed.
References
Könönen, E., Gursoy, M. & Gursoy, U. K. Periodontitis: a multifaceted disease of tooth-supporting tissues. J. Clin. Med. 8, 1135 (2019).
Bardají, D. K. R. et al. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 40, 18–27 (2016).
Diaz, P. I., Hoare, A. & Hong, B. Y. Subgingival microbiome shifts and community dynamics in periodontal diseases. J. Calif. Dent. Assoc. 44, 421–435 (2016).
Lamont, R. J. & Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 21, 172–183 (2015).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725 (2012).
How, K. Y., Song, K. P. & Chan, K. G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 7, 53 (2016).
Holt, S. C., Kesavalu, L., Walker, S. & Genco, C. A. Virulence factors of Porphyromonas gingivalis. Periodontology 2000(20), 168–238 (1999).
Hajishengallis, G. & Lamont, R. J. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 44, 328–338 (2014).
Figuero, E. et al. Quantification of periodontal pathogens in vascular, blood, and subgingival samples from patients with peripheral arterial disease or abdominal aortic aneurysms. J. Periodontol. 85, 1182–1195 (2014).
Konig, M. F. et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 8, 369ra176 (2016).
Bao, K. et al. Aggregatibacter actinomycetemcomitans H-NS promotes biofilm formation and alters protein dynamics of other species within a polymicrobial oral biofilm. NPJ Biofilms Microbiomes. 4, 12 (2018).
Ando, E. S. et al. Immune response to cytolethal distending toxin of Aggregatibacter actinomycetemcomitans in periodontitis patients. J. Periodontal Res. 45, 471–480 (2010).
Henderson, B., Ward, J. M. & Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen?. Periodontol 2000(54), 78–105 (2010).
Kittichotirat, W., Bumgarner, R. E., Asikainen, S. & Chen, C. Identification of the pangenome and its components in 14 distinct Aggregatibacter actinomycetemcomitans strains by comparative genomic analysis. PLoS ONE 6, e22420 (2011).
Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins (Basel). 3, 242–259 (2011).
Linhartová, I. et al. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34, 1076–1112 (2010).
Aimetti, M. Nonsurgical periodontal treatment. Int. J. Esthet. Dent. 9, 251–267 (2014).
Marcinkiewicz, J., Strus, M. & Pasich, E. Antibiotic resistance: a “dark side” of biofilm associated chronic infections. Pol. Arch. Med. Wewn. 123, 309–313 (2013).
Caetano da Silva, S. D. et al. Antibacterial activity of Pinus elliottii against anaerobic bacteria present in primary endodontic infections. Anaerobe 30, 146–152 (2014).
Leandro, L. M. et al. Chemistry and biological activities of terpenoids from Copaiba (Copaifera spp.) Oleoresins. Molecules 30, 3866–3889 (2012).
Arruda, C. et al. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus-a review. Biomed. Pharmacother. 109, 1–20 (2019).
Souza, A. B. et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf Against Cariogenic Bacteria. Phytother. Res. 25, 215–220 (2011).
Souza, A. B. et al. Antimicrobial evaluation of diterpenes from Copaifera langsdorffii oleoresin against periodontal anaerobic bacteria. Molecules 16, 9611–9619 (2011).
Abrão, F. et al. Copaifera langsdorffii oleoresin and its isolated compounds: antibacterial effect and antiproliferative activity in cancer cell lines. BMC Comp. Altern. Med. 15, 443 (2015).
Leandro, L. F. et al. Assessment of the antibacterial, cytotoxic and mutagenic potential of the phenolic-rich hydroalcoholic extract from Copaifera trapezifolia Hayne leaves. J. Med. Microbiol. 65, 937–950 (2016).
Moraes, T. S. et al. In vitro evaluation of Copaifera oblongifolia oleoresin against bacteria causing oral infections and assessment of its cytotoxic potential. Curr. Pharm. Biotechnol. 17, 894–904 (2016).
Borges, C. H. et al. Copaifera duckei oleoresin and its main nonvolatile terpenes: vitro schistosomicidal properties. Chem. Biodivers. 13, 1348–1356 (2016).
Alves, J. M. et al. Copaifera multijuga oleoresin and its constituent diterpene (-)-copalic acid: Genotoxicity and chemoprevention study. Mutat. Res. 819, 26–30 (2017).
Abrão, F. et al. Antibacterial effect of Copaifera duckei Dwyer oleoresin and its main diterpenes against oral pathogens and their cytotoxic effect. Front. Microbiol. 9, 201 (2018).
Furtado, R. A. et al. Assessment of toxicogenetic activity of oleoresins and leaves extracts of six Copaifera species for prediction of potential human risks. J. Ethnopharmacol. 15, 119–125 (2018).
Souza, M. G. M. et al. ent-copalic acid antibacterial and anti-biofilm properties against Actinomyces naeslundii and Peptostreptococcus anaerobius. Anaerobe 52, 43–49 (2018).
da Silva, J. J. M. et al. Development of a validated ultra-high-performance liquid chromatography tandem mass spectrometry method for determination of acid diterpenes in Copaifera oleoresins. J. Chromatogr. A. 1515, 81–90 (2017).
Carneiro, L. J. et al. Development and validation of a rapid and reliable RP-HPLC-PDA method for the quantification of six diterpenes in Copaifera duckei, Copaifera reticulata and Copaifera multijuga Oleoresins. J. Braz. Chem. Soc. 4, 729–737 (2018).
Carneiro, L. J. et al. Copaifera multijuga, Copaifera pubiflora and Copaifera trapezifolia oleoresins: chemical characterization and in vitro cytotoxic potential against tumoral cell lines. J. Braz. Chem. Soc. 8, 1679–1689 (2020).
Esfahani, Z. J., Kadkhoda, Z., Eshraghi, S. S. & Surmaghi, M. H. S. Antibacterial Effect of an Herbal Product Persica on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: an in-vitro study. J. Dent. (Tehran). 11, 464–472 (2014).
Wu, Y., Yan, J., Chen, L. & Gu, Z. Association between infection of different strains of Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival plaque and clinical parameters in chronic periodontitis. J. Zhejiang Univ. Sci. B. 8, 121–131 (2007).
Kikuchi, Y. et al. Porphyromonas gingivalis mutant defective in a putative extracytoplasmic function sigma factor shows a mutator phenotype. Oral Microbiol. Immunol. 24, 377–383 (2009).
Fujise, K., Kikuchi, Y., Kokubu, E., Okamoto-Shibayama, K. & Ishihara, K. Effect of extracytoplasmic function sigma factors on autoaggregation, hemagglutination, and cell surface properties of Porphyromonas gingivalis. PLoS ONE 12, e0185027 (2017).
Chen, T., Yong, R., Dong, H. & Duncan, M. J. A general method for direct sequencing of transposon mutants by randomly primed PCR. Tech. Tips Online. 4, 58–61 (1999).
Chen, T., Dong, H., Yong, R. & Duncan, M. J. Pleiotropic pigmentation mutants of Porphyromonas gingivalis. Microb. Pathog. 28, 235–247 (2000).
Lima, F. L. et al. Leukotoxic activity of Actinobacillus actinomycetemcomitans isolated from human and non-human primates. Braz. J. Microbiol. 32, 250–256 (2001).
Verdonk, M. L. et al. Modeling water molecules in protein−ligand docking using GOLD. J. Med. Chem. 48, 6504–6515 (2005).
Jones, G., Willett, P., Glen, R. C., Leach, A. R. & Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 267, 727–748 (1997).
Ríos, J. L. & Récio, M. C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 100, 80–84 (2005).
Gibbons, S. Phytochemicals for bacterial resistance–strengths, weaknesses and opportunities. Planta Med. 74, 594–602 (2008).
Tincusi, B. M. et al. Antimicrobial terpenoids from the oleoresin of the peruvian medicinal plant Copaifera paupera. Planta Med. 68, 808–812 (2002).
Bakri, I. M. & Douglas, C. W. Inhibitory effect of garlic extract on oral bacteria. Arch. Oral Biol. 50, 645–651 (2005).
Alves, F. R. F., Silva, M. G., Rôças, I. N. & Siqueira Júnior, J. F. Biofilm biomass disruption by natural substances with potential for endodontic use. Braz. Oral. Res. 27, 20–25 (2013).
Fux, C. A., Stoodley, P., Hall-Stoodley, L. & Costerton, J. W. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti. Infect. Ther. 1, 667–683 (2003).
Kuriyama, T., Karasawa, T., Nakagawa, K., Nakamura, S. & Yamamoto, E. Antimicrobial susceptibility of major pathogens of orofacial odontogenic infections to 11 β-lactam antibiotics. Oral Microbiol. Immunol. 17, 285–289 (2002).
Gaetti-Jardim, E. Jr. et al. Distribution of biotypes and leukotoxic activity of Aggregatibacter actinomycetemcomitans isolated from Brazilian patients with chronic periodontitis. Braz. J. Microbiol. 39, 658–663 (2008).
Sebaugh, J. L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 10, 128–134 (2011).
Park, J. H., Lee, J. K., Um, H. S., Chang, B. S. & Lee, S. Y. A periodontitis-associated multispecies model of an oral biofilm. J. Periodontal. Implant. Sci. 44, 79–84 (2014).
Gabrilska, R. A. & Rumbaugh, K. P. Biofilm models of polymicrobial infection. Fut. Microbiol. 10, 1997–2015 (2015).
Inoshita, E. et al. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect. Immun. 52, 421–427 (1986).
DeCarlo, A. A., Paramaesvaran, M., Yun, P. L., Collyer, C. & Hunter, N. Porphyrin-mediated binding to hemoglobin by the HA2 domain of cysteine proteinases (gingipains) and hemagglutinins from the periodontal pathogen Porphyromonas gingivalis. J. Bacteriol. 181, 3784–3791 (1999).
Dixon, D. R., Jeffrey, N. R., Dubey, V. S. & Leung, K. P. Antimicrobial peptide inhibition of Porphyromonas gingivalis 381: induced hemagglutination is improved with a synthetic decapeptide. Peptides 30, 2161–2167 (2009).
Senhorinho, G. N. et al. Occurrence and antimicrobial susceptibility of Porphyromonas spp. and Fusobacterium spp. in dogs with and without periodontitis. Anaerobe 18, 381–385 (2012).
Wahasugui, T. C., Nakano, V., Piazza, R. M. & Avila-Campos, M. J. Phenotypic and genotypic features of Aggregatibacter actinomycetemcomitans isolated from patients with periodontal disease. Diagn. Microbiol. Infect. Dis. 75, 366–372 (2013).
Nakagawa, I. et al. Identification of a new variant of fimA gene of Porphyromonas gingivalis and its distribution in adults and disabled populations with periodontitis. J. Periodontal. Res. 37, 425–432 (2002).
Meyer, D. H., Lippman, J. E. & Fives-Taylor, P. M. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic multistep process. Infect. Immun. 64, 2988–2997 (1996).
Löhr, G. et al. Polyphenols from Myrothamnus flabellifolia Welw: inhibit in vitro adhesion of Porphyromonas gingivalis and exert anti-inflammatory cytoprotective effects in KB cells. J. Clin. Periodontol. 38, 457–469 (2011).
Fitzpatrick, R. E., Wijeyewickrema, L. C. & Pike, R. N. The gingipains: scissors and glue of the periodontal pathogen: Porphyromonas gingivalis. Fut. Microbiol. 4, 471–487 (2009).
Li, N. & Collyer, C. A. Gingipains from Porphyromonas gingivalis - Complex domain structures confer diverse functions. Eur. J. Microbiol. Immunol. (Bp). 1, 41–58 (2011).
Eichinger, A. Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 18, 5453–5462 (1999).
Guevara, T. et al. Structural determinants of inhibition of Porphyromonas gingivalis gingipain K by KYT-36, a potent, selective, and bioavailable peptidase inhibitor. Sci. Rep. 9, 4935 (2019).
Shen, A. Allosteric regulation of protease activity by small molecules. Mol. Biosyst. 6, 1431 (2010).
Lally, E. T., Hill, R. B., Kieba, I. R. & Korostoff, J. The interaction between RTX toxins and target cells. Trends Microbiol. 7, 356–361 (1999).
Kaplan, J. B., Schreiner, H. C., Furgang, D. & Fine, D. H. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40, 1181–1187 (2002).
Fine, D. H. et al. A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J. Clin. Microbiol. 51, 2850–2861 (2013).
Konopka, K., Pretzer, E., Felgner, P. L. & Düzgüneş, N. Human immunodeficiency virus type-1 (HIV-1) infection increases the sensitivity of macrophages and THP-1 cells to cytotoxicity by cationic liposomes. Biochim. Biophys. Acta. 1312, 186–196 (1996).
Acknowledgements
We gratefully acknowledge the financial support of the Brazilian Funding Agencies São Paulo Research Foundation (FAPESP), Grants # 2016/23012-2, # 2011/13630-7 and 2018/21537-6, and National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Contributions
A.F.: contributed to data acquisition and interpretation and manuscript drafting. S.T.S.: contributed to data acquisition; and performed all the statistical analyses. M.C.L.: contributed to data acquisition and critically revised the manuscript. A.S.R. and V.R.C.S.: contributed to interpretation, manuscript drafting, and critical review of the manuscript. P.R.E.F.: contributed with data acquisition and interpretation and revision of the final version of the manuscript. B.J.K. and M.C.H.G. contributed to conception, design, data acquisition and interpretation, manuscript drafting, and critical review of the manuscript. All the authors gave their final approval and agreed to be accountable for all the aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abrão, F., Silva, T.S., Moura, C.L. et al. Oleoresins and naturally occurring compounds of Copaifera genus as antibacterial and antivirulence agents against periodontal pathogens. Sci Rep 11, 4953 (2021). https://doi.org/10.1038/s41598-021-84480-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84480-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.