Abstract
Aging is a natural and progressive process characterized by an increased frequency of age-related diseases such as cancer. But its mechanism is unclear. TNFAIP8L2 (Tipe2) is an important negative regulator for homeostasis through inhibiting TLR and TCR signaling. Our work reveals that Tipe2 might have dual function by regulating senescence. One side, the overexpression of Tipe2 in CRC cells could induce typical senescent phenotype, especially exposure to oxidative stress. Tipe2 inhibits telomerase activity by regulating c-Myc and c-Est-2 binding to the hTERT promotor. Interestingly, Tipe2 KO mice treated with D-Gal showed a less serious inverse of CD4:CD8 ratio, a lower percentage of Treg compared to WT. Besides, Tipe2 KO mice were more tolerant to the initiation of AOM/DSS-induced CRC, accompanied by a lower level of Treg within IEL. Therefore, specific antibodies against CD25 effectively ameliorate tumorigenesis. These data suggest strongly that the overexpressed Tipe2 suppresses tumor cells proliferation and survival, but endogenous Tipe2 promotes the initiation of tumorigenesis when exposure to dangerous environment such as AOM/DSS-related inflammation.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death worldwide [1]. Studies demonstrated that inflammatory bowel diseases (IBD) including ulcerative colitis (UC) and Crohn’s disease were chronic inflammatory disorders of the gastrointestinal tract, strongly suggesting association with an increased risk of CRC development [2,3,4]. Recent studies showed that CRC might be related to cellular senescence [5, 6]. While the precise mechanism remains unknown.
Cellular senescence and telomere attrition are important processes which are involved in aging [7]. The decline of immune function is thought to be tightly associated with age-related diseases, such as cancer [8,9,10]. The hallmarks of immune senescence include an inverse of CD4/CD8 ratio, a shift from naïve to memory T cell phenotype, poor T-cell proliferative responses to stimuli, an increase of pro-inflammatory cytokines (such as IL-1, IL-6, TNF-α), these age-related changes result in the failure of homeostasis [11,12,13,14]. On the other hand, replicative senescence can be triggered by telomere shortening which is repaired and maintained by the activity of telomerase [15, 16]. However, the senescent cells induced by both replicative and cellular senescence are characterized by an enlarged and flattened shape, and generate specific biomarkers, such as an irreversible cell cycle arrest in the G0/G1 phase, increased content of β-galactosidase, overexpression of P21 and P16, etc. [17,18,19]. Substantial evidences have shown that blocking senescence accelerates [5] and induction of senescence inhibits CRC development [6]. Therefore, senescence might be a potential target for tumor therapy.
Tumor necrosis factor-α induced protein 8 like-2 (TNFAIP8L2, Tipe2) is the one member of the Tipe (TNFAIP8) family which can function as transfer proteins for phosphoinositide second messengers [20,21,22]. They are regulators for homeostasis, both in inflammation and carcinogenesis [22,23,24]. Their abnormal expressions are observed in various human diseases [21, 25,26,27]. Tipe2-deficient cells are hypersensitive to stimuli and defective in polarization and chemotaxis [20, 21] and Tipe2-deficient mice are resistant to leukocyte mediated inflammation [27]. In addition to negatively regulating pathogen-induced immune response, Tipe2 in DCs is also capable of promoting immune response under homeostatic conditions through the suppression of peripheral tolerance [25]. However, it is not clear if Tipe2 plays any role in senescence.
In the current study, we investigated the roles of Tipe2 in senescence using a colitis-associated CRC model and D-Gal-induced aging model. The results demonstrated that Tipe2 might play dual function in CRC by inducing senescence: suppresses the proliferation and survival of tumor cells but accelerates the initiation of AOM/DSS-induced CRC.
Results
Tipe2-deficiency resists aging, while the overexpression in CRC cells promotes cellular senescence
To determine the roles of Tipe2 in senescence, we detected sera biochemical parameters from C57BL/6 (WT) and TIPE2-/- mice of different ages. The results showed that although the sera levels of albumin (ALB) decreased markedly both in WT and Tipe2-deficient mice with aging, the levels were significantly higher in Tipe2-/- mice of 12 m than that in matched WT (Fig. S1A). The total plasma levels of alkaline phosphatase (ALP, Fig. S1B), alanine aminotransferase (ALT, Fig. S1C), glutamic oxaloacetylase (ASTL, Fig. S1D), cholesterol (CHO, Fig. S1E), and GGT (Fig. S1F) were upregulated significantly both in WT and Tipe2-deficient mice with aging, but were much lower in Tipe2-deficient mice (Fig. S1). Importantly, the levels of Tipe2 protein were much higher in WT mice of 24 m than in 3 m ones (Fig. 1K). These data suggested that Tipe2 might be associated with aging in mice.
The apoptotic cells were increased (A), while the cell proliferation (B) was inhibited in Tipe2 transfected HT-29 cells. C, the cell cycle progression was inhibited in Tipe2 transfected HT-29 or ASMC-7 cells. D Tipe2 expression in HT-29 cells. E caspase 3 (33kD) decreased, while P21 increased in Tipe2 overexpression HT-29 or ASMC-7 cells. F Tipe2 inhibits NF-κB signaling pathway. Tipe2 expression was higher in the 7th than in 14th passage AMSC cells (G). H H2O2 stimulation upregulated the Tipe2 expression in HT-29 cells. I, Sa-β-gal positive cells were increased in HT-29 cells transfected with Tipe2 plasmid with or without H2O2 stimulation. J Sa-β-gal positive cells were increased in primary cultured AMSC transfected with Tipe2 plasmid with or without H2O2 stimulation. K the levels of Tipe2 protein in WT mice of 24 months were significantly increased than that in 3 months ones. Data are representative of three independent experiments and expressed as means ± SEM. Significant difference between two groups was determined using an unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001. Data from A and C were collected using BECKMAN COULTER CytoFLEXS, then analyzed using CytExpert.
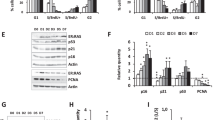
Since senescent cells have typical features, such as an ability of lower proliferation and cell cycle arrest, we transfected Tipe2 plasmid to CRC cells and primary cells to determine the roles of Tipe2 in cell senescence. As shown in Fig. 1, Tipe2 can promote cell death and inhibit cell proliferation. The overexpression of Tipe2 in HT-29 cells significantly induced cell apoptosis, especially exposure to ROS stimuli (Fig. 1A and D). The cell growth was markedly suppressed (Fig. 1B) resulting from an inhibition of NF-κB signaling (Fig. 1F) [20], therefore the cell cycle arrested at the G0/G1 phase both in HT-29 and primary cultured 7th ASMC cells transfected with Tipe2 plasmid (Fig. 1C). This phenomenon was confirmed by the increased expression of the P21 protein, and downregulation of the pro-caspase 3 (33 kd, Fig. 1E) both in CRC cells and primary cells. The upregulation of P21 is considered as an important marker for cellular senescence [28], suggesting that Tipe2 might promote cellular senescence.
Increased level of reactive oxygen species (ROS) generation is closely associated with cellular senescence [29]. Therefore, ROS (H2O2) stimulation and senescence-associated β-galactosidase (SA-β-Gal) staining were used to confirm the effect of Tipe2 on cellular senescence. Normally, the level of Tipe2 protein was much higher in the 7th passage AMSC cells than in that 14th cells (Fig. 1G). Tipe2 expression was upregulated significantly when cells exposure to H2O2 stimulation (Fig. 1H). The ratio of SA-β-Gal staining positive cells in pRK5-Tipe2 transfected cells was much higher than that in pRK5 controls (Fig. 1I), especially exposure to H2O2 stimulation, suggesting that ROS stimuli markedly increased the number of senescent cells induced by Tipe2 overexpression (Fig. 1I). The same phenomena were observed in primary cultured cells (Fig. 1J). These data indicated that Tipe2 might promote cellular senescence, especially exposure to oxidative stress.
Tipe2 promotes replicative senescence by regulating telomerase activity
Replicative senescence can be triggered by the shortening of chromosome ends (also called telomeres which are regulated by telomerase activity). To explore the role of Tipe2 on telomere, the telomerase activity was determined in Tipe2 overexpressed cells. The results showed that the overexpression of Tipe2 in HT-29 and SW480 cells could inhibit the expression of hTERT (Fig. 2A). Accordingly, the telomerase activity decreased significantly (Fig. 2B). c-Myc is an important regulator for telomerase activity [30]. We found that the overexpression of Tipe2 in tumor cells markedly decreased the protein level of c-Myc, but increased the phosphorylation level of p-Smad3 (Fig. 2C). Further studies showed that Tipe2 downregulated the cytoplasm c-Myc, but did not affect nucleus c-Myc (Fig. 2D). Very interestingly, the level of nucleus Tipe2 was significantly higher compared with control cells (Fig. 2D). While IP results showed that Tipe2 couldn’t bind to c-Myc (Fig. 2E), suggesting that Tipe2 downregulated the expression of c-Myc, but did not affect its nucleus translocation. Figure 2F showed that the effect of Tipe2 on c-Myc downregulation might be associated with the inhibition of the phosphorylation of ERK. But the inhibitor SGH772984 for ERK didn’t suppress the effect of Tipe2 on hTERT, suggesting that might have other factors regulate hTERT activity through Tipe2.
A the overexpression of Tipe2 in HT-29 and SW480 cells inhibited the expression of hTERT. B the telomerase activity was significantly decreased in Tipe2 transfected HT-29 and SW480 cells. C Tipe2 overexpression in HT-29 cells downregulated c-Myc protein while upregulated the phosphorylation level of p-Smad3. Tipe2 downregulated the cytoplasm c-Myc but not nucleus c-Myc (D), and didn’t bind to c-Myc (E). F Tipe2 inhibited the phosphorylation of ERK and the expression of c-Myc. Tipe2 inhibited c-Ets-2 expression (G), bound to cytoplasmic c-Ets-2 (I) resulting a decreased level of the nucleus c-Ets-2 (H) in HT-29 cells. The images were analyzed using ImageJ software.
c-Ets-2, a genuine cancer-specific transcription factor, upregulates the telomerase activity by binding to the promoter of hTERT [31]. As expected, the overexpression of Tipe2 markedly downregulated the c-Ets-2 expression (Fig. 2G). Interestingly, Tipe2 did not affect cytoplasm c-Ets-2, but decreased its nucleus level (Fig. 2H). Further IP experiment showed that Tipe2 blocked c-Ets-2 translocation from cytoplasm to nucleus by binding to c-Ets-2 in the cytoplasm (Fig. 2I, Fig. S2), suggesting that Tipe2 might suppress telomerase activity by blocking the translocation of c-Ets-2.
Tipe2-deficiency is resistance to aging in the D-gal-induced mice
D-Galactose (D-Gal), a reducing sugar normally present in the body, is widely used to induce an ideal model to study the possible mechanism of aging-related disease [32]. To address the role of Tipe2 in aging, Tipe2-/- mice and WT controls were administrated with D-Gal to induce aging models (Fig. 3K). WT mice treated with D-Gal showed more severe age-related losses in villous and enterocyte heights, a decreasing number of goblet cells, and the depth of crypt in the intestinal morphology (Fig. 3B and D). While there was no significant difference between WT and Tipe2-/- mice without D-Gal treatment (Fig. 3A and C). Besides, the microglial cells were reduced in length, showed less branching and vacuole formation in WT mice treated with D-Gal compared to matched Tipe2-/- mice, especially neuron cells showed more serious apoptotic or aging-related morphology including chromatic agglutination, karyopyknosis, nuclear fragmentation, and cytoplasmic vacuolation (Fig. 3F and H). But there was no significant difference between WT and Tipe2-deficient mice without D-Gal (Fig. 3E and G).
The HE staining of intestinal tissues (A–D) and brain (E–H) from D-Gal induced mice models. Without D-Gal the intestinal or brain from WT (A, E) and Tipe2-/- (C, G) showed no difference. Severe age-related morphology was observed in intestinal and brain from WT mice (B, F) treated with D-Gal compared to Tipe2-deficient mice (D, H). Tipe2 KO mice treated with D-Gal showed a less serious inverse of CD4:8 ratio (I) and a lower percentage of Tregs (J) compared to WT controls. K The procedure of D-Gal induced aging mouse model. Data are representative of three independent experiments and expressed as means ± SEM. Significant difference between two groups was determined using an unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001. Data from I and J were collected using BECKMAN COULTER CytoFLEXS, then analyzed using CytExpert.
Oxidative stress plays an important role in inducing brain aging. Superoxide dismutase (SOD), an important enzyme involved in the removal of ROS, and monoamine oxidase (MAO, a brain regional mitochondrial enzyme), malondialdehyde (MDA, an end-product of ROS-induced peroxidation), are widely used as the oxidative stress biomarkers [33]. In this study, D-Gal could downregulate the level of SOD in serum both in WT and Tipe2-/- mice, but the SOD level from Tipe2-/- mice treated with D-Gal was much higher (Fig. S3A). On the other hand, the levels of MDA (Fig. S3B) and MAO (Fig. S3C) were significant higher in D-Gal treated both WT and Tipe2 KO mice, while the latter were much lower, suggesting that Tipe2-deficient mice might resist D-Gal induced aging through resisting oxidative stress.
The inverted CD4:8 ratio and the upregulation of Tregs are considered as aging-associated immune markers [34]. In this study, we found that Tipe2 KO mice treated with D-Gal showed a less serious inverse of CD4:8 ratio. Although there was no significant difference about the baseline ratio, D-Gal could significantly downregulate the ratio both in Tipe2-/- mice and WT controls, but it was significantly higher in D-Gal-treated Tipe2-/- mice (Fig. 3I). Furthermore, the percentage of Tregs in Tipe2-/- mice was significantly lower than that in WT without D-Gal treatment (Fig. 3J). D-Gal could upregulate Tregs levels both in Tipe2-/- mice and matched WT, but the level was much lower in Tipe2-/- mice (Fig. 3J). These results suggested that Tipe2-deficiency might resist immune senescence in D-Gal-induced mice.
Tipe2-deficiency in naïve CD4+ T cells might inhibit iTreg differentiation
We found that the levels of Tregs from Tipe2-/- mice treated with D-Gal were significantly lower compared with WT/D-Gal mice (Fig. 3J). Liu R et al. reported that Tipe2 in DC could inhibit the induction of pTregs in the gut mucosa [35]. To verify its role in Treg differentiation, naïve CD4+ T cells separated from Tipe2-/- or C57 mice were cultured with TGF-β to induce Tregs (iTregs) [36]. The results showed that TGF-β upregulated the levels of iTreg markedly both in Tipe2-/- and Tipe2+/+ naïve CD4+ T (Fig. 4A), but it was much lower in Tipe2-/- ones (Fig. 4A). Results from WB revealed that Tipe2-deficiency could increase the level of p-Akt phosphorylation in TGF-β activated naïve CD4+ T cells, while decrease the phosphorylation of p-Smad3 (Fig. 4B). The activation of TGF-β/Smad signaling pathway is associated with the increase in senescent phenotype [37]. Therefore, to confirm our results, TGF-β/Smad3 signaling pathway was detected in primary and tumor cell lines transfected with or without Tipe2 plasmid. We found that the overexpression of Tipe2 upregulated the expression of TGF-β protein both in primary cultured 7th ASMC cells and CRC cells (Fig. 4C), accompanied by the upregulation of p-Smad3 (Fig. 2C). The activation of the Smad3 pathway and the inhibition of Akt-signaling are considered the most important signaling pathways during iTreg differentiation [38]. These data indicated that Tipe2 might enhance senescence through promoting the naïve CD4+ T cells to differentiate into iTregs with TGF-β stimuli.
A Showed a lower percentage of iTregs differentiated from Tipe2-deficent naïve CD4+ T cells in vitro. Data were collected using BECKMAN COULTER CytoFLEXS, then analyzed using CytExpert. B Showed an increased level of p-Akt and decreased p-Smad3 in Tipe2-deficent naïve CD4+ T cells with TGF-β stimulation. The images were analyzed using ImageJ software. C The overexpression of Tipe2 upregulated TGF-β expression in HT-29 or AMSC cells. Data are representative of three independent experiments and expressed as means ± SEM. Significant difference between two groups was determined using an unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Tipe2-deficient mice are higher resistance to AOM/DSS-induced tumorigenesis
AOM is a potential DNA damage-inducing agent that is commonly used to induce colorectal cancer (CRC). To investigate the role of Tipe2 in colitis-associated CRC, we used a protocol that combines the AOM carcinogen with DSS-induced colitis (Fig. 5A) [39]. We found that the total tumor number of AOM/DSS-induced CRC from Tipe2+/+ mice was higher than that from Tipe2-/- (Fig. 5B), especially in tumors with greater diameter more than 2 mm (Fig. 5C), suggesting that the occurrence of AOM/DSS-induced CRC in Tipe2+/+ mice was probably earlier than that in Tipe2-/- mice. The Tipe2+/+ mice showed more severe inflammation with many areas of crypt lost (Fig. 5D). They presented widespread mucous glands destruction and derangement, epithelial atypia proliferation, while Tipe2 knockout could alleviate cancer atypia and inflammation. The length of the colon from Tipe2+/+ mice was significantly shorter than Tipe2-/- ones (Fig. 5E). Inflammatory mediators are upregulated during AOM/DSS-induced CRC, but Tipe2-/- mice showed lower serum levels of inflammatory cytokines, such as IL-6 (Fig. 5F), MCP-1 (Fig. 5G), IL-12 (Fig. 5H), and TNF-α (Fig. 5I). The changes of these cytokines, especially IL-6, are consistent with the notion that IL-6 is critical for colon tumor development. These data suggested that Tipe2 might promote the initiation of AOM/DSS-induced CRC.
A The protocol of AOM/DSS-induced CRC. The tumor number was significant lower in Tipe2-deficent AOM/DSS models (B), especially tumors less than 2 mm in diameter (C). Tipe2-defiency showed less severe intestinal morphology (D) accompanied by longer length of the colon (E) in AOM/DSS-induced mice. Tipe2-deficent AOM/DSS models showed lower serum levels of proinflammatory cytokines, such as IL-6 (F), MCP-1 (G), IL-12 (H) and TNF-α (I). Data are representative of three independent experiments and expressed as means ± SEM. Significant difference between two groups was determined using an unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Tipe2-deficiency was more susceptible to anti-CD25-induced Treg depletion
To confirm the role of Tipe2 on iTreg differentiation, anti-CD25 mAb was used to block the effect of Treg in AOM/DSS-induced colon cancer. The used protocol was shown in Fig. 6A. As expected, the total CRC number from AOM/DSS/anti-CD25 treated mice were much lower than that from the mice without anti-CD25 injection (Fig. 6B), especially tumors with size more than 2 mm (Fig. 6C, left panel). Very interestingly, the tumor number from anti-CD25 treated Tipe2-/- mice was significantly lower compared with anti-CD25 treated Tipe2+/+ controls (Fig. 6B), especially tumors with size more than 2 mm (Fig. 6C, left panel). There was no significant difference in tumor number with size less than 2 mm between Tipe2+/+ and Tipe2-/- mice with or without anti-CD25 treatment (Fig. 6C, right panel). Tissues from antibody administrated Tipe2+/+ mice showed more serious inflammatory morphology compared with matched Tipe2-/- mice, but the severity of the inflammation and injury of AOM/DSS induced CRC from two groups was ameliorated effectively after anti-CD25 injection (Fig. 6D, E and F). BrdU labeling showed more positive cells (tumor cells) in AOM/DSS treated WT compared to matched Tipe2 KO mice, but markedly decreased in anti-CD25 injected mice (Fig. 6G). WB results showed that the levels of c-Myc, activation of ERK and NF-κB in AOM/DSS-treated WT models were much higher than that in Tipe2-deficient models (Fig. S4).
A The used protocol in the experiment. The total tumor number (B) or tumors less than 2 mm in diameter (C) was significant lower in Tipe2-deficent AOM/DSS models, especially in models with anti-CD25 treatment. Tipe2-defiency showed less severe intestinal inflammation (D) accompanied by longer length of the colon (E and F) in AOM/DSS models, especially with anti-CD25 treatment. G The BrdU positive cells decreased in Tipe2-deficent AOM/DSS models, especially with anti-CD25 treatment. Significant difference between two groups was determined using an unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Results of flow cytometry analysis showed that AOM/DSS treatment upregulated the percentage of CD25+FoxP3+ T cell (Treg) in IEL separated from both Tipe2-/- mice and Tipe2+/+ (Fig. 7A), but was significantly lower in AOM/DSS-induced Tipe2-/- mice (Fig. 7A). With anti-CD25 treatment, the percentage of Treg decreased significantly in both two groups (Fig. 7A). Very importantly, the anti-CD25-treated Tipe2-/- mice showed a much lower Treg ratio (Fig. 7A) and a decrease in the serum level of TGF-β (Fig. 7B), but did not affect the levels of IL-17 (Fig. 7C). These data suggested that the anti-CD25 might ameliorate AOM/DSS-associated CRC via blocking CD25+Foxp3+ Treg differentiation, especially in Tipe2-deficient mice.
A The percentage of Tregs from IEL separated from Tipe2-defienct AOM/DSS models was significant lower than WT controls, especially with anti-CD25 treatment. Data were collected using BECKMAN COULTER CytoFLEXS, then analyzed using CytExpert. Tipe2-deficent AOM/DSS models showed lower serum levels of TGF-β (B) and IL-17 (C) with or without anti-CD25 antibody treatment. D Tipe2 inhibits tumor cell growth while promotes the initiation of AOM/DSS-induced colon cancer through regulating senescence.
Discussion
Our work reveals that Tipe2 might have dual function by regulating senescence: overexpression in tumor cells inhibits tumor cell proliferation and survival, while endogenous Tipe2-deficiency suppresses colitis-associated colorectal cancer (CRC) initiation. One side, the overexpression of Tipe2 in CRC cells suppresses cell growth, promotes cell cycle arrest, inhibits telomerase activity by regulating transcription factors, such as c-Myc and c-Est-2, which bind to the hTERT promotor. The percentage of SA-β-Gal staining positive cells (senescent cells) is increased in Tipe2 overexpression cells, especially exposure to oxidative stress. This is accompanied by a less serious inverse of CD4: CD8 ratio, a lower percentage of Treg in PBMC from Tipe2 KO mice treated with D-Gal compared to matched WT. Besides, Tipe2-deficiency is more tolerant to the initiation of AOM/DSS-induced CRC. This is accompanied by a lower level of Treg within IEL from the AOM/DSS-treated Tipe2 KO mice. Therefore, specific antibodies against CD25 effectively ameliorate tumorigenesis. These data suggest strongly that through inducing senescence, Tipe2 suppresses tumor cells proliferation and survival, but promotes the initiation of tumorigenesis when exposure to dangerous environment such as AOM/DSS-related inflammation.
In agreement with large amounts of previous evidence for the suppressor role of Tipe2 in tumor cells [21,22,23], we demonstrate that the overexpression in CRC cells can inhibit cell growth, upregulates P21 expression and promotes cell cycle arrest in G0/G1 phase, which are considered to be senescent phenotype. The phenomenon might result from that Tipe2 involved in replicative senescence by regulating hTERT. Replicative senescence can be triggered by the shortening of chromosome ends (also called telomeres, which are regulated by telomerase activity). hTERT is the main catalytic unit for telomerase activity. We found that the overexpression of Tipe2 markedly downregulated the expression of c-Myc and c-Ets-2, which are important regulators for hTERT transcription [30, 31]. Tipe2 also blocks the nucleus translocation of c-Ets-2. Accordingly, the telomerase activity decreased significantly. Cells with Tipe2 overexpression are inclined to senescent phenotype. These data further confirm the notion that the overexpression of Tipe2 plays suppressor role in tumor cells [20,21,22].
However, the existence of Tipe2 might be favorable to the initiation of AOM/DSS induced CRC. Present papers published on Tipe2 and colon cancer suggest Tipe2 a tumor suppressor but very few experiments to verify [23, 40, 41], especially no AOM/DSS CRC model. The AOM/DSS protocol is highly dependent on DSS which induces epithelial inflammation and apoptosis. Consistent with Lou’s report that Tipe2-deficiency suppressed the DSS-associated colitis in mouse model [42], we reported that AOM/DSS-treated Tipe2 KO mice exhibited significantly less severe colitis and this might result in less severe tumorigenesis. This was accompanied by a lower percentage of CD4+CD25+Foxp3+ Treg cells within IEL, suggesting that Tipe2 might be associated with Treg differentiation.
CD4+CD25+ Tregs are instrumental in the maintenance of immunological tolerance. Several reports revealed that Tipe2 was associated with the suppressive function of tTregs [43, 44]. It promoted the thymus egress of tTregs and did not affect tTregs development [45]. But its expression in DC could inhibit the induction of pTregs in the gut mucosa [35]. Therefore, to confirm the role of Tipe2 in Treg function and development, the naïve peripheral CD4+CD25- T cells were separated from Tipe2 KO mice and WT. These cells were induced to differentiate into CD4+CD25+Foxp3+ Tregs (iTreg) through co-stimulation with TCR and TGF-β [36]. We found that Tipe2-deficient naïve CD4+ T cells were less susceptible to differentiate into iTregs through regulating TGF-β/Smad signaling pathway in vitro, but more susceptible to the Tregs depletion induced by anti-CD25 antibody in vivo.
The suppressive mechanisms of Treg on immune response might be associated with Treg-induced effector T-cell senescence [46]. We found that D-Gal-treated Tipe2-deficient mice showed a less serious reverted CD4:8 ratio accompanied by a downregulation of Treg percentage. Very interestingly, AOM/DSS-treated Tipe2-deficient mice showed less severe inflammation and tumorigenesis, especially exposure to anti-CD25 depletion. These data indicated that AOM/DSS-treated Tipe2-deficient mice showed less severe CRC might due to the suppression of iTreg differentiation induced by Tipe2 in naïve CD4+ T cells. Targeting Treg-induced effector T-cell senescence might be a checkpoint for immunotherapy against cancer and other age-related diseases.
Tipe2-deficient mice were less susceptible to D-Gal-induced aging. Tipe2 KO mice showed less severe age-related intestinal and brain morphology and lower serum levels of oxidative stress biomarkers, such as MDA and MAO, while a higher level of SOD which plays important role in the removal of ROS. Very interestingly, a less serious inverted CD4:8 ratio and lower percentage of Tregs, which are considered as aging-related immune markers [34], were observed in D-Gal-treated Tipe2-deficient mice. These results suggested that Tipe2-deficiency might be resistant to D-Gal-induced senescence in mice.
Taken together, our work reveals a dual function of Tipe2 on AOM/DSS-induced CRC through promoting senescence. Tipe2-deficiency is less susceptible to senescence and might be a checkpoint for immunotherapy against cancer and other age-related diseases.
Materials and methods
Antibodies
The following antibodies or reagents used for flow cytometry were from Biolegend: FITC anti-mouse CD4 (Cat#100509), PE anti-mouse CD25 (Cat#102007), Alexa Fluor® 647 anti-mouse FOXP3 (Cat#126407), FITC anti-mouse CD3 (Cat#100203) or PerCP/Cy5.5 anti-mouse CD3ε (Cat#100327), APC anti-mouse CD8a (Cat#100711), TruStain fcX™ (anti-mouse CD16/32, Cat#101320) and True-Nuclear™ Transcription Factor Buffer Set (Cat#424401) was used for blocking non-specific binding of immunoglobulin to the Fc receptors and intracellular staining perm wash buffer, respectively. Antibodies to hTERT (Cat#ab230527, 1:1000 dilution) was from Abcam, ERK (Cat#4694 T, 1:1000 dilution), p-ERK (Cat#4376 T, 1:1000 dilution), Akt (Cat#2920 T, 1:1000 dilution), p-Akt (Ser473) (Cat#9271 S, 1:1000 dilution), p-Smad3 (Cat#9513 T) and Smad3 (Cat#9520 T, WB 1:1000 dilution; IF 1:50 dilution), c-Myc (Cat#9402 S, WB 1:1000 dilution; IF 1:50 dilution), c-Ets-2 (Cat#66476 S, WB 1:1000 dilution; IF 1:50 dilution), IκB (Cat#9242 T, 1:1000 dilution), p-IκB (Cat#9246 T, 1:1000 dilution), caspase-3 (Cat#9662 T, 1:1000 dilution), P21 (Cat#37543, 1:1000 dilution), were from Cell Signaling Technology (CST). Antibodies to β-actin (Cat#66009-1-Ig, 1:1000 dilution), β-tubulin (Cat#66240-1-Ig, 1:1000 dilution), LaminB1 (Cat#66095-1-Ig, 1:1000 dilution), and TGF-β (Cat#21898-1-AP, 1:1000 dilution) were from Proteintech, CD3ε and CD28 (Cat#114.52D/114.53D) were from Invitrogen.
AOM/DSS colitis-associated carcinoma mouse model
The Tipe2-deficient (Tipe2−/−) mice (C57BL/6 J background) have been described previously [20]. C57BL/6 J mice (WT, Tipe2+/+) were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Science. All mice used were male and 6-7 weeks old, randomly divided into groups and no blinding was used. Mice were housed under pathogen-free conditions of humidity (50 ± 10%), lighting (12 h light/dark cycle), and temperature (25 ± 2 °C) with pure water and a freely accessible pelleted basal diet in the University of Shandong Animal Care Facilities. All handlings and procedures were carried out as per the protocol approved by the Ethical Committee of the School of Basic Medical Science, Shandong University.
For colitis-associated carcinoma models, pilot studies were used for estimation of the sample size required to ensure adequate power. Tipe2-deficient mice and WT controls were randomly divided into four groups (five per experimental group): Tipe2 KO treated with AOM/DSS or saline control, WT treated with AOM/DSS or saline control. In the first week the test groups were injected intraperitoneally with a single-dose of azoxymethane (AOM, Cat#A5486, Sigma-Aldrich, St. Louis, MO, 10 mg/kg). After 5 days, dextran sodium sulfate (DSS, 2%, Sigma-Aldrich, Cat#160110) was dissolved in drinking water and administrated to mice for 5 days, followed by 14 days of regular drinking water. The protocol was described in Fig. 5A. The same protocol was performed with intraperitoneal normal saline and drinking distilled water instead of the AOM/DSS treatment in the control groups. For Treg depletion model, Tipe2 KO mice and WT were randomly divided into six groups (six per experimental group): Tipe2 KO treated with AOM/DSS/anti-CD25 antibody, AOM/DSS or saline control, WT treated with AOM/DSS/anti-CD25 antibody, AOM/DSS or saline control. The depletion model mice were treated with anti-CD25 antibodies (Sungenebiotec, China) as described in Fig. 6A. The body weight and health conditions of the mice were recorded every week. On the last night before mice were sacrificed, animals and their respective controls were injected intraperitoneally with BrdU (5-bromo-2′-deoxyuridine, 10 mg/kg, Sigma-Aldrich, Cat#19-160) to label newly born cells. Animals were sacrificed 100 days after the AOM injection. Colons were excised and flushed with cold PBS, size measurements were performed using a digital caliper in a blinded fashion. Then the colons were fixed in 10% formalin solution (Sigma) at room temperature, and paraffin-embedded for HE staining. All data were expressed as mean ± SEM. Differences between two groups were determined as unpaired two-tailed Student’ s t-test. P < 0.05 was considered as statistically significant.
Aging animal model
Male WT and Tipe2 -/- mice were sacrificed at the age of 3 (n = 3) and 24 months (n = 3). Fresh sera were collected to determine albumin (ALB), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (ASTL), gamma glutamyl transpeptidase (GGT), and total cholesterol (CHO) using commercial kits (Changchun huili, C061/C063; Jiubang, CK-E28753) according to the manufacturer’s instructions. One part of the tissues such as spleen was frozen in −80 °C to detect Tipe2 protein using western blot.
Senescent Tipe2-deficient (n = 6) and WT (n = 6) mice were induced by single-dose intraperitoneal injection of D-Galactose (D-Gal, 500 mg/kg, Sigma-Aldrich, Cat#G0750) every day for 2 months and were sacrificed in the 14th week, and the brain, large bowels were excised for evaluating histological evidence. Fresh sera were collected to determine Superoxide dismutase (SOD) (Cat#BC0170, Solarbio, Beijing, China), monoamine oxidase (MAO) (Cat#K009679P, Solarbio), Malondialdehyde (MDA) (Cat#BC0025, Solarbio). Peripheral blood was collected to detect CD4+ T cells, CD8+ T cells, and Tregs by Flow cytometry.
Hematoxylin–eosin (HE) staining and histological analysis
The formalin-fixed colon tissues were embedded in paraffin blocks. Sliced sections about 4 mm were deparaffinized and rehydrated by a xylene–ethanol–water gradient system. Hematoxylin and eosin (HE) staining was performed followed by a dehydrating process. Histopathological examination was performed under a light microscope by Olympus (Waltham, MA, USA). Neoplasms and inflammations were analyzed and diagnosed as the established criteria. Histopathological evaluation was determined by two pathologists from the pathology department of Qilu Hospital (Dr. Chao Ma and Dr. Chunyan Hao, Shandong, China) who were not aware of the experimental protocols.
Detection of inflammatory cytokines
ELISA kit was used to detect the expression of several inflammatory cytokines such as MCP-1 (Cat#432704, Biolegend), IL-17A (Cat#433007, Biolegend), TGF-β (Cat#432507, Biolegend), IL-6 (Cat#1210602, DAKEWE), IL-12p70 (Cat#1211202, DAKEWE) and TNFα (Cat#1217202, DAKEWE) in the sera of mice models. All analyses and calibrations were performed in duplicate. Optical densities were determined using an absorbance microplate reader (Tecan, Infinite M200, Switzerland) at 450 nm. Graph prism 8 Data Analysis software was used to analyze all data. Differences between two groups were determined as unpaired two-tailed Student’ s t-test.
The separation of Tregs from IEL
According to the procedure described in [47], the Tregs were separated from IEL. The colon was removed from the mouse model and washed in ice-cold RPMI (HyClone, USA). IEL was isolated and transfered to a clean tube. Flow cytometry was performed to analyze Tregs from IEL.
iTreg differentiation
6–8 weeks male Tipe2 KO and WT mice (five per experimental group) were sacrificed and spleens were collected into sterile complete RPMI. Single cell suspensions were obtained by mashing the spleen and passing cells through 70 μm cell strainer (BD, Franklin Lakes NJ, USA). Naïve CD4+ T cells were obtained according to the protocol of Miltenyi. 24 well sterile tissue culture plates (Corning, NY, USA) were coated with 4 μg/mL anti-CD3ε and 4 μg/mL anti-CD28 in 0.5 mL/well PBS at 37 °C for 2 h. Purified naïve CD4+ T cells which was isolated from Tipe2-/- or WT spleen were washed and resuspended in complete RPMI media supplemented with 100 IU/mL rhIL-2 (Peprotech, Cat#AF-200-02-10, Rocky Hill, NJ, USA) and 5 ng/mL rhTGF-β (Peprotech, Cat#100-21C-2). 3 × 105 cells were added to the wells at a volume of 1 mL/well. Cells were cultured at 37 °C 5% CO2 for 3 days and harvested for flow cytometry.
Flow cytometry
Surface markers were stained in PBS with antibodies for 20 min at room temperature, the cells were fixed in Cytoperm/Cytofix (Cat#88-8824-00, Invitrogen, USA), permeabilized with Perm/Wash Buffer (Invitrogen) and stained with Biolegend conjugated antibodies. iTreg stained with FITC-anti-CD4, PE-anti-CD25 and APC-anti-Foxp3a. Cell cycle and apoptosis were determined in HT-29 and ASMC cells transfected with Tipe2-pRK5 or vector control. Data were collected using BECKMAN COULTER CytoFLEXS, then analyzed using CytExpert.
Cell culture
Colon cancer cell line (HT-29, TCHu103) obtained from National Collection of Authenticated Cell Culture (NCACC) and primary lung airway smooth muscle cells in mice (ASMC, CP-M005) obtained from Procell Life Science & Technology were cultured in the RIPA1640 (HyClone) medium containing 10% FBS (HyClone). SW480 (TCHu172) obtained from NCACC was cultured in L-15 medium containing 10% FBS. The cells were incubated in wet cell incubator with 5% CO2 at 37 °C. The expression of Tipe2 was detected using western blot in the 7th generation and 14th generation ASMC cells. Tipe2-pRK5 plasmid was transfected into ASMC or HT-29 cells treated with or without H2O2 (Sigma-Aldrich, Cat#88597). SA-β-Gal (Byeotime, Cat#C0602, Shanghai, China) staining was used to detect cell senescence. The relationship between the levels of Tipe2 protein and cell senescence was analyzed.
Proliferation analysis
The cleaved protein of caspase-3 (CST, USA) and P21 (CST) were detected using western blot, the cells proliferation was detected using CCK-8 kit (APExBIO, Cat#K1018, USA). About 1 × 104 HT-29 cells transfected with pRK5-Tipe2 or vector control were cultured in a 96-well plate. When reaching 90% confluence, cells were treated with or without H2O2 for different times. After treatment with 10 μL of CCK-8 reagent for 2 h, absorbance was determined at 450 nm. The experiments were performed with six replicated wells per sample, and the assays were conducted in triplicate.
Telomerase activity analysis
The activity of telomerase was detected by TeloTAGGG Telomerase PCR ELISA Kit (Roche Diagnostics GMBH Mannheim, Cat#11854666910). The relationship between the levels of Tipe2 protein and hTERT activity is analyzed.
Cytoplasmic and nuclear protein extraction
Cell cytoplasm and nucleus protein were collected with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) according to the manufactures’ protocol. The distributions of protein in cytosol and nucleus were analyzed by western blot.
Co-immunoprecipitation assay
After the cultured cells washed with pre-chilled PBS for 2 times, these cells were added in cold RIPA lysis buffer. After centrifuge, the supernatant (cell extract) was transferred to a new tube for IP assay. Firstly, about 100 μg cell extract was pre-cleared with protein A agarose (Sigma-Aldrich, Cat#IP02), then incubated with anti-Tipe2 (1:200, JinSiTe, Nanjing, China) antibody at 4 °C overnight with constant rotation. Then, the protein/antibody mixture was incubated with the pretreated Protein A-sepharose beads (Sigma-Aldrich). After the precipitant was collected and washed, the beads were resuspended in SDS-PAGE loading buffer and heated at 95 °C for 5 min. The supernatant was analyzed by SDS-PAGE and western blotting.
Western blot
50 μg protein was separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore). Tipe2 was detected using a specific antibody as previously described [48]. The following primary antibodies were used: anti-ERK and p-ERK, anti-Akt and p-Akt, anti-p-Smad and anti-Smad (CST), anti-c-Myc and anti-c-Ets-2; anti-β-actin (Proteintech). Immunoblotting was conducted by incubating the membranes with primary antibodies at 4 °C overnight followed by secondary antibodies (goat anti-rabbit Ig G or goat anti-mouse Ig G, 1:5000) conjugated with peroxidase for 1 h at room temperature. After washing, bound peroxidase activity was detected by the ECL detection system (ECL, F-cheiBIsi.6pro, DNR) using the Super Signal West Pico kit (Pierce Biotechnology). The images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
All data were expressed as mean ± SEM (standard error of the mean). Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software Inc. San Diego, CA). Significant difference between two groups was determined using an unpaired two-tailed Student’s t-test. P < 0.05 was considered as statistically significant.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30.
Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22:4794–801.
Rivera ED, Coffey JC, Walsh D, Ehrenpreis E. The mesentery, systemic inflammation, and Crohn’s disease. Inflamm Bowel Dis 2019;25:226–34.
Stidham RW, Higgins P. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg 2018;31:168–78.
Hou Z, Guo K, Sun X, Hu F, Chen Q, Luo X, et al. TRIB2 functions as novel oncogene in colorectal cancer by blocking cellular senescence through AP4/p21 signaling. Mol Cancer 2018;17:172–86.
Huang Y, Lei J, Yi G, Huang F, Li Y, Wang C, et al. Coroglaucigenin induces senescence and autophagy in colorectal cancer cells. Cell Prolif 2018;51:e12451.
Lopez-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech Ageing Dev 2017;162:108–21.
Giunco S, Petrara MR, Bergamo F, Bianco PD, Zanchetta M, Carmona F, et al. Immune senescence and immune activation in elderly colorectal cancer patients. Aging. 2019;11:3864–75.
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell 2014;159:709–13.
Wang Y, Lee A, Lu L, Ke L, Chen W, Dong J, et al. Human electronegative LDL induces mitochondrial dysfunction and premature senescence of vascular cells in vivo. Aging Cell 2018;17:e12792.
Ray D, Yung R. Immune senescence, epigenetics and autoimmunity. Clin Immunol 2018;196:59–63.
Froy H, Sparks AM, Watt K, Sinclair R, Bach F, Pilkington JG, et al. Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science 2019;365:1296–8.
Xu W, Larbi A. Markers of T cell senescence in humans. Int J Mol Sci 2017;18:1742–56.
Thomas R, Wang W, Su DM. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing 2020;17:2–18.
Stögbauer L, Stummer W, Senner V, Brokinkel B. Telomerase activity, TERT expression, hTERT promoter alterations, and alternative lengthening of the telomeres (ALT) in meningiomas. Neurosurg Rev 2020;43:903–10.
Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet 1997;17:498–502.
Dodig S, Čepelak I, Pavić I. Hallmarks of senescence and aging. Biochem Med. 2019;29:030501–15.
Frenk S, Houseley J. Gene expression hallmarks of cellular ageing. Biogerontology 2018;19:547–66.
Lauri A, Pompilio G, Capogrossi MC. The mitochondrial genome in aging and senescence. Ageing Res Rev 2014;18:1–15.
Sun H, Gong S, Caimody RJ, Hilliard A, Li L, Sun J, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 2008;133:415–26.
Fayngerts SA, Wang Z, Zamani A, Sun H, Boggs AE, Porturas TP, et al. Direction of leukocyte polarization and migration by the phosphoinositide-transfer protein TIPE2. Nat Immunol 2017;18:1353–60.
Goldsmith JR, Chen YH. Regulation of inflammation and tumorigenesis by the TIPE family of phospholipid transfer proteins. Cell Mol Immunol 2017;14:482–7.
Padmavathi G, Banik K, Monisha J, Bordoloi D, Shabnam B, Arfuso F, et al. Novel tumor necrosis factor-α induced protein eight (TNFAIP8/TIPE) family: Functions and downstream targets involved in cancer progression. Cancer Lett 2018;432:260–71.
Wu X, Kong Q, Zhan L, Qiu Z, Huang Q, Song X. TIPE2 ameliorates lipopolysaccharide‑induced apoptosis and inflammation in acute lung injury. Inflamm Res 2019;68:981–92.
Liu R, He X, Geng W, Wang T, Ruan Q. Loss of TIPE2 has opposing effects on the pathogenesis of autoimmune diseases. Front Immunol 2019;10:2284–96.
Shi G, Zhao J, Sun X, Ma J, Wang P, He F, et al. TIPE2 is negatively correlated with tissue factor and thrombospondin-1 expression in patients with bronchial asthma. Exp Ther Med 2018;15:3449–54.
Yan D, Wang J, Sun H, Zamani A, Zhang H, Chen W, et al. TIPE2 specifies the functional polarization of myeloid-derived suppressor cells during tumorigenesis. J Exp Med 2020;217:e20182005.
Hsu C, Altschuler SJ, Wu LF. Patterns of early p21 dynamics determine proliferation-senescence cell fate after chemotherapy. Cell 2019;178:361–73.
Lu T, Finkel T. Free radicals and senescence. Exp Cell Res 2008;314:1918–22.
Nakamura M, Hayashi M, Konishi H, Nunode M, Ashihara K, Sasaki H, et al. MicroRNA-22 enhances radiosensitivity in cervical cancer cell lines via direct inhibition of c-Myc binding protein, and the subsequent reduction in hTERT expression. Oncol Lett 2020;19:2213–22.
Hsu C, Lee L, Tang S, Hsin I, Lin Y, Ko J. Epidermal growth factor activates telomerase activity by direct binding of Ets-2 to hTERT promoter in lung cancer cells. Tumor Biol 2015;36:5389–98.
Sun K, Yang P, Zhao R, Bai Y & Guo Z. Matrine attenuates D-Galactose-induced aging-related behavior in mice via inhibition of cellular senescence and oxidative stress. Oxid Med Cell Longev. 2018;2018:7108604.
Wang L, Chen Q, Zhuang S, Wen Y, Cheng W, Zeng Z, et al. Effect of anoectochilus roxburghii flavonoids extract on H2O2-induced oxidative stress in LO2 cells and D-gal induced aging mice model. J Ethnopharmacol 2020;254:112670–83.
Dillon SM, Liu J, Purba CM, Christians AJ, Kibbie JJ, Castleman MJ, et al. Age-related alterations in human gut CD4 T cell phenotype, T helper cell frequencies, and functional responses to enteric bacteria. J Leukoc Biol 2020;107:119–32.
Liu R, Liu C, Liu C, Fan T, Geng W, Ruan Q. TIPE2 in dendritic cells inhibits the induction of pTregs in the gut mucosa. Bio Bio Res Commu 2019;509:911–7.
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–86.
Chen W, Shin K, Kim S, Shon W, Kim RH, Park N, et al. hTERT peptide fragment GV1001 demonstrates radioprotective and antifibrotic effects through suppression of TGF-β signaling. Inter J Mol Med. 2018;41:3211–20.
Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 2008;9:632–40.
Gupta J, Barrantes I, Igea A, Sakellariou S, Pateras I, Gorgoulis VG, et al. Dual Function of p38a MAPK in colon cancer: Suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell. 2014;25:1–17.
Zhong M, et al. Expression of TIPE family members in human colorectal cancer. Oncol Lett 2021;21:118–28.
Niture S, et al. Oncogenic Role of Tumor Necrosis Factor α-Induced Protein 8(TNFAIP8). Cells. 2018;8:9–24.
Lou Y, Sun H, Morrissey S, Porturas T, Liu S, Hua X, et al. Critical roles of TIPE2 protein in murine experimental colitis. J Immunol 2014;193:1064–70.
Ding J, Su J, Zhang L, Ma J. Crocetin activates Foxp3 through TIPE2 in asthma-associated Treg cells. Cell Physiol Biochem 2015;37:2425–33.
Luan Y, Yao Y, Zhang L, Dong N, Zhang Q, Yu Y, et al. Expression of tumor necrosis factor-α induced protein 8 like-2 contributes to the immunosuppressive property of CD4(+)CD25(+) regulatory T cells in mice. Mol Immunol 2011;49:219–26.
Fan T, Huang X, Liu C, Liu R, Wang T, Ruan Q. Egress of murine regulatory T cells from the thymus requires TIPE2. Biochem Biophys Res Commun 2018;500:376–83.
Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh E, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun 2018;9:249–64.
Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol 2011;12:312–9.
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang Y, et al. Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol 2010;47:2435–42.
Acknowledgements
We would like to thank Dr. Jing Liu (Shandong University) for her kindly help in statistical analysis and Dr. Youhai H. Chen (Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences) for insightful advice. FCM was performed in Advanced Medical Research Institute, Shandong University.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No.81873864), the National Outstanding Youth Fundation of China (No.81525012), and the National Natural Science Fundation (Key Program) (No.31730026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All handlings and procedures described in this manuscript were approved by the Ethical Committee of the School of Basic Medical Science, Shandong University.
Consent for publication
All authors have provided their consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Availability of data and materials
The data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Edited by Professor Hans-Uwe Simon
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Zhang, N., Ma, C. et al. The overexpression of Tipe2 in CRC cells suppresses survival while endogenous Tipe2 accelerates AOM/DSS induced-tumor initiation. Cell Death Dis 12, 1001 (2021). https://doi.org/10.1038/s41419-021-04289-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-021-04289-0