Abstract
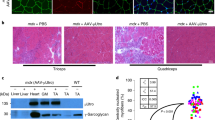
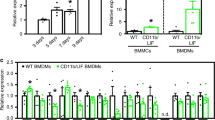
A significant problem affecting gene therapy approaches aiming at achieving long-term transgene expression is the immune response against the protein product of the therapeutic gene, which can reduce or eliminate the therapeutic effect. The problem is further exacerbated when therapy involves targeting an immunogenic tissue and/or one with a pre-existing inflammatory phenotype, such as dystrophic muscles. In this proof-of-principle study, we co-expressed a model antigen, bacterial β-galactosidase, with an immunosuppressive factor, indoleamine 2,3-dioxygenase 1 (IDO1), in muscles of the mdx mouse model of Duchenne muscular dystrophy. This treatment prevented loss of expression of the transgene concomitant with significantly elevated expression of T-regulatory (Treg) markers in the IDO1-expressing muscles. Moreover, co-expression of IDO1 resulted in reduced serum levels of anti-β-gal antibodies. These data indicate that co-expression of genes encoding immunomodulatory enzymes controlling kynurenine pathways provide a viable strategy for preventing loss of transgenes targeted into dystrophic muscles with pre-existing inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lavigne MD, Pohlschmidt M, Novo JF, Higgins B, Alakhov V, Lochmuller H et al. Promoter dependence of plasmid-pluronics targeted alpha galactosidase A expression in skeletal muscle of Fabry mice. Mol Ther 2005; 12: 985–990.
Wells KE, Maule J, Kingston R, Foster K, McMahon J, Damien E et al. Immune responses, not promoter inactivation, are responsible for decreased long-term expression following plasmid gene transfer into skeletal muscle. FEBS Lett 1997; 407: 164–168.
Marino M, Scuderi F, Provenzano C, Bartoccioni E . Skeletal muscle cells: from local inflammatory response to active immunity. Gene Ther 2011; 18: 109–116.
Blake DJ, Weir A, Newey SE, Davies KE . Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 2002; 82: 291–329.
Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW . Immune-mediated mechanisms potentially regulate the disease time-course of duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R 2009; 1: 755–768.
Cirak S, Feng L, Anthony K, Arechavala-Gomeza V, Torelli S, Sewry C et al. Restoration of the dystrophin-associated glycoprotein complex after exon skipping therapy in Duchenne muscular dystrophy. Mol Ther 2012; 20: 462–467.
Aoki Y, Yokota T, Wood MJ . Development of multiexon skipping antisense oligonucleotide therapy for Duchenne muscular dystrophy. Biomed Res Int 2013; 2013: 402369.
Malik V, Rodino-Klapac LR, Viollet L, Mendell JR . Aminoglycoside-induced mutation suppression (stop codon readthrough) as a therapeutic strategy for Duchenne muscular dystrophy. Ther Adv Neurol Disord 2010; 3: 379–389.
Goyenvalle A, Seto JT, Davies KE, Chamberlain J . Therapeutic approaches to muscular dystrophy. Hum Mol Genet 2011; 20: R69–R78.
Mendell J, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S et al. Transient expression of a therapeutic dystrophin transgene in Duchenne muscular dystrophy revealed by T cell mediated immunity. Neurology 2010; 74 A111.
Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM . Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA 1995; 92: 1401–1405.
Wang Z, Storb R, Halbert CL, Banks GB, Butts TM, Finn EE et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther 2012; 20: 1501–1507.
Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang LX, Herzog RW . Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA 2006; 103: 4592–4597.
Buhlmann JE, Foy TM, Aruffo A, Crassi KM, Ledbetter JA, Green WR et al. In the absence of a CD40 signal, B cells are tolerogenic. Immunity 1995; 2: 645–653.
Dobrzynski E, Herzog RW . Tolerance induction by viral in vivo gene transfer. Clin Med Res 2005; 3: 234–240.
Ezzelarab M, Thomson AW . Tolerogenic dendritic cells and their role in transplantation. Semin Immunol 2011; 23: 252–263.
Mascarell L, Zimmer A, Van Overtvelt L, Tourdot S, Moingeon P . Induction of allergen-specific tolerance via mucosal routes. Curr Top Microbiol Immunol 2011; 352: 85–105.
Gravano DM, Vignali DA . The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci 2012; 69: 1997–2008.
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011; 12: 870–878.
Madoiwa S, Yamauchi T, Hakamata Y, Kobayashi E, Arai M, Sugo T et al. Induction of immune tolerance by neonatal intravenous injection of human factor VIII in murine hemophilia A. J Thromb Haemost 2004; 2: 754–762.
Zhang J, Xu L, Haskins ME, Parker Ponder K . Neonatal gene transfer with a retroviral vector results in tolerance to human factor IX in mice and dogs. Blood 2004; 103: 143–151.
Min B, Legge KL, Caprio JC, Li L, Gregg R, Zaghouani H . Differential control of neonatal tolerance by antigen dose versus extended exposure and adjuvant. Cell Immunol 2000; 200: 45–55.
Ichino M, Mor G, Conover J, Weiss WR, Takeno M, Ishii KJ et al. Factors associated with the development of neonatal tolerance after the administration of a plasmid DNA vaccine. J Immunol 1999; 162: 3814–3818.
Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA 2012; 109: 2497–2502.
Hara M, Yokoyama H, Fukuyama K, Kitamura N, Shimokawa N, Maeda K et al. Transcriptional regulation of the mouse CD11c promoter by AP-1 complex with JunD and Fra2 in dendritic cells. Mol Immunol 2013; 53: 295–301.
Ni J, Nolte B, Arnold A, Fournier P, Schirrmacher V . Targeting anti-tumor DNA vaccines to dendritic cells via a short CD11c promoter sequence. Vaccine 2009; 27: 5480–5487.
Edelmann SL, Nelson PJ, Brocker T . Comparative promoter analysis in vivo: identification of a dendritic cell-specific promoter module. Blood 2011; 118: e40–e49.
Young CN, Sinadinos A, Lefebvre A, Chan P, Arkle S, Vaudry D et al. A novel mechanism of autophagic cell death in dystrophic muscle regulated by P2RX7 receptor large-pore formation and HSP90. Autophagy 2015; 11: 113–130.
Wolff J, Malone R, Williams P, Chong W, Acsadi G, Jani A et al. Direct gene transfer into mouse muscle in vivo. Science 1990; 247 (4949 Pt 1): 1465–1468.
Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 2009; 325: 1142–1146.
Jung ID, Lee MG, Chang JH, Lee JS, Jeong YI, Lee CM et al. Blockade of indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J Immunol 2009; 182: 3146–3154.
Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK . Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res 2010; 70: 129–138.
Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clement M et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab 2015; 22: 460–471.
Nitahara-Kasahara Y, Hayashita-Kinoh H, Chiyo T, Nishiyama A, Okada H, Takeda S et al. Dystrophic mdx mice develop severe cardiac and respiratory dysfunction following genetic ablation of the anti-inflammatory cytokine IL-10. Hum Mol Genet 2014; 23: 3990–4000.
Hedrich CM, Bream JH . Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol Res 2010; 47: 185–206.
Hsieh C, Macatonia S, Tripp C, Wolf S, O'Garra A, Murphy K . Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993; 260: 547–549.
Eickhoff CS, Schnapp AR, Sagartz JE, Hoft DF . Lethal NK-mediated inflammation induced by IL-12 in the absence of polymorphic and nonpolymorphic MHC class I molecules. Cytokine 2013; 64: 25–29.
Matino D, Gargaro M, Santagostino E, Di Minno MND, Castaman G, Morfini M et al. IDO1 suppresses inhibitor development in hemophilia A treated with factor VIII. J Clin Invest 125: 3766–3781.
Shinde R, Shimoda M, Chaudhary K, Liu H, Mohamed E, Bradley J et al. B cell-intrinsic IDO1 regulates humoral immunity to T cell-independent antigens. J Immunol 2015; 195: 2374–2382.
Vavrincova-Yaghi D, Deelman LE, van Goor H, Seelen MA, Vavrinec P, Kema IP et al. Local gene therapy with indoleamine 2,3-dioxygenase protects against development of transplant vasculopathy in chronic kidney transplant dysfsunction. Gene Ther 2016; 23: 797–806.
Sinadinos A, Young CN, Al-Khalidi R, Teti A, Kalinski P, Mohamad S et al. P2RX7 purinoceptor: a therapeutic target for ameliorating the symptoms of Duchenne muscular dystrophy. PLoS Med 2015; 12: e1001888.
Lavigne MD, Yates L, Coxhead P, Gorecki DC . Nuclear-targeted chimeric vector enhancing nonviral gene transfer into skeletal muscle of Fabry mice in vivo. FASEB J 2008; 22: 2097–2107.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9: 676–682.
Aubert D, Menoret S, Chiari E, Pichard V, Durand S, Tesson L et al. Cytotoxic immune response blunts long-term transgene expression after efficient retroviral-mediated hepatic gene transfer in rat. Mol Ther 2002; 5: 388–396.
Acknowledgements
We thank Professor J Golab, Drs C Sharp and T Brocker for their kind gifts of pcDNA3.IDO1, lacZ, and CD11c promoter plasmids, respectively and Dr P Kalinski for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, D., Al-Khalidi, R., Edgar, S. et al. Co-delivery of indoleamine 2,3-dioxygenase prevents loss of expression of an antigenic transgene in dystrophic mouse muscles. Gene Ther 24, 113–119 (2017). https://doi.org/10.1038/gt.2016.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.82