Abstract
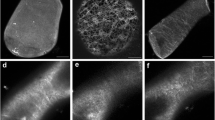
We studied the effects of the anti-microtubule drug, oryzalin, on the content and spatial organization of reticuloplasmins (Ca2+-binding marker proteins of the endoplasmic reticulum) in winter wheat seedlings after their cold acclimation (3°C, 7 days) and treatment with ABA (30 μM). For identification and visualization of reticuloplasmins, we applied one-dimensional SDS-PAGE with subsequent Western blotting and indirect fluorescent microscopy. We used polyclonal HSP70 and CRH antibodies against BiP and calreticulin (Cal), respectively. On immunoblots, the brightest bands corresponded to polypeptides with mol wts of 58 kD (calreticulin) and 79 kD (BiP). The content of calreticulins in roots was shown to be higher than in leaves. Cold acclimation enhanced, and ABA treatment reduced, the concentration of calreticulins in root cells. Both treatments increased the BiP concentration in roots. Oryzalin (10 μM, treatment for 3 h) did not affect the level of reticuloplasmins in roots of unhardened, cold acclimated, treated with ABA and with a combination of cold and ABA plants. However, both oryzalin and low-temperature treatments resulted in the accumulation of reticuloplasmins in the two spherical structures in the vicinity of the plasmalemma and nuclear envelope. After the combined action of oryzalin and low temperature, the cortical sphere of BiP proteins was shifted into the endoplasm and calreticulins appeared in the nuclear matrix. We believe that these changes in the reticuloplasmin localization are related to the rearrangement of the endoplasmic reticulum determined by the cytoskeleton modification. They result in the improved capacity of reticuloplasmins to control Ca2+ behavior and/or to the function as chaperones. The results obtained permit the conclusion that cytoskeletal proteins interact with reticuloplasmins, and this interaction might be involved in the transduction of the external and internal signals.
Similar content being viewed by others
REFERENCES
Macer, D.R.J. and Koch, G.L.E., Identification of a Set of Calcium Binding Proteins in Reticuloplasm, the Luminal Content of the Endoplasmic Reticulum, J. Cell Sci., 1988, vol. 92, pp. 61-70.
Waser, M., Mesaeli, N., Spencer, C., and Michalak, M., Regulation of Calreticulin Gene Expression by Calcium, J. Cell Biol., 1997, vol. 138, pp. 547-557.
Denecke, J., Carlsson, L.E., Vidal, S., Hoglund, A., Ek, B., Zeijl, M.J., Sinjorgo, K.M.C., and Palva, T., The Tobacco Homolog of Mammalian Calreticulin Is Present.202 in Protein Complexes in vivo, Plant Cell, 1995, vol. 7, pp. 391-406.
Michalak, M., Mariani, P., and Opas, M., Calreticulin, a Multifunctional Ca2+ Binding Chaperone of the Endoplasmic Reticulum, Biochem. Cell Biol., 1998, vol. 76, pp. 779-785.
Mery, L., Mesaeli, N., Michalak, M., Opas, M., Lew, D.P., and Krause, K.H., Overexpression of Calreticulin Increases Intracellular Ca2+ Storage and Decreases Store-Operated Ca2+ Influx, J. Biol. Chem., 1996, vol. 271, pp. 9332-9339.
Dedhar, S., Rennie, P.S., Shago, M., Leung-Hagesteijn, C.Y., Filmus, J., Hawley, R.G., Bruchovsky, N., Cheng, H., Matusik, R.J., and Giguere, V., Inhibition of Nuclear Hormone Receptor Activity by Calreticulin, Nature, 1994, vol. 367, pp. 480-483.
Sapiro, R.G., Zhu, Q., Bhoyroo, V., and Soling, H.D., Definition of the Lectin-Like Properties of the Molecular Chaperone, Calreticulin, and Demonstration of Its Copurification with Endomannosidase from Rat Liver Golgi, J. Biol. Chem., 1996, vol. 271, pp. 11588-11594.
Kubawara, K., Pinsky, D.J., Sciacca, R., Benedict, C., Brett, J., Ogawa, S., Broekman, M.J., Marcus, A.J., Sciacca, R., and Michalak, M., Calreticulin, an Antithrombotic Agent which Binds to Vitamin K+-Dependent Coagulation Factors, Stimulates Endothelial Nitric Oxide Production, and Limits Thrombosis in Canine Coronary Arteries, J. Biol. Chem., 1995, vol. 270, pp. 8179-8187.
Singh, N.K., Atreya, C.D., and Nakhasi, H.L., Identification of Calreticulin as a Rubella Virus RNA Binding Protein, Proc. Natl. Acad. Sci. USA, 1994, vol. 91, pp. 12770-12774.
Sontheimer, R.D., Lieu, T.S., and Capra, J.D., Calreticulin: The Diverse Functional Repertoire of a New Human Autoantigen, Immunologist, 1993, vol. 1, pp. 155-160.
Wang, X., Sato, R., Brown, M.C., Hau, X., and Goldstein, J.L., SREBP-1, a Membrane-Bound Transcription Factor Released by Sterol-Regulated Proteolysis, Cell, 1994, vol. 77, pp. 53-62.
Burns, K., Duggan, B., Atkinson, A.E., Famulski, K.S., Nemer, M., Bleackley, R.C., and Michalak, M., Modulation of Gene Expression by Calreticulin Binding to the Glucocorticoid Receptor, Nature, 1994, vol. 367, pp. 476-480.
Lievremont, J.P., Rizzuto, R., Hendershot, L., and Meldolesi, J., BIP, a Major Chaperone Protein of the Endoplasmic Reticulum Lumen, Plays a Direct and Important Role in the Storage of the Rapidly Exchanging Pool of Ca2+, J. Biol. Chem., 1997, vol. 272, pp. 30873-30879.
Anderson, J.V., Li, Q.B., and Guy, C.L., Structural Organization of the Spinach Endoplasmic Reticulum Luminal 70-Kilodalton Heat-Shock Cognate Gene and Expression of 70-Kilodalton Heat-Shock Genes during Cold Acclimation, Plant Physiol., 1994, vol. 104, pp. 1459-1370.
Coppolino, M.G. and Dedhar, S., Ligand-Specific, Transient Interaction between Integrins and Calreticulin during Cell Adhesion to Extracellular Matrix Proteins Is Dependent upon Phosphorylation/Dephosphorylation Events, Biochem. J., 1999, vol. 340, pp. 41-50.
Mogelsvang, S. and Simpson, P.J., Changes in the Levels of Seven Proteins Involved in Polypeptide Folding and Transport during Endosperm Development of Two Barley Genotypes Differing in Storage Protein Localization, Plant Mol. Biol., 1998, vol. 36, pp. 541-552.
Nick, P., Signals, Motors, Morphogenesis-the Cytoskeleton in Plant, Development Plant Biol., 1999, vol. 1, pp. 169-179.
Morejohn, L.C., Bureau, T.E., Mole-Bajer, J., Bajer, A.S., and Fosket, D.E., Oryzalin, a Dinitroaniline Herbicide, Binds to Plant Tubulin and Inhibits Microtubule Polymerization in vitro, Planta, 1987, vol. 172, pp. 252-264.
Raudaskoski, M., Aström, H., Perttila, K., Virtanen, I., and Louhelainen, J., Role of the Microtubule Cytoskeleton in Pollen Tubes: An Immunocytochemical and Ultrastructural Approach, Biol. Cell, 1987, vol. 61, pp. 177-188.
Baluška, F., Parker, J.S., and Barlow, P.W., Specific Patterns of Cortical and Endoplasmic Microtubules Associated with Cell Growth and Tissue Differentiation in Roots of Maize (Zea mays), J. Cell Sci., 1992, vol. 103, pp. 191-200.
Bradford, M., A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem., 1976, vol. 72, pp. 248-254.
Roderick, L., Llewellyn, D.H., Campbell, A.K., and Kendall, J.M., Role of Calreticulin in Regulating Intracellular Ca 2+ Storage and Capacitative Ca 2+ Entry in HeLa Cells, Cell Calcium, 1998, vol. 24, pp. 253-262.
Guy, C.L., Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1990, vol. 41, pp. 187-223.
Kolesnichenko, A.V., Pobezhimova, T.P., and Voinikov, V.K., Cold-Shock Proteins in Plants, Fiziol. Rast. (Moscow), 2000, vol. 47, pp. 624-630 (Russ. J. Plant Physiol., Engl. Transl.).
Monroy, A.F., Castonguay, Y., Laberge, S., Sarhan, F., Vezina, L.P., and Dhindsa, R.R., A New Cold-Induced Alfalfa Gene Is Associated with Enhanced Hardening at Subzero Temperature, Plant Physiol., 1993, vol. 102, pp. 873-879.
Veisz, O., Galiba, G., and Sutka, J., Effect of Abscisic Acid on the Cold Hardiness of Wheat Seedlings, J. Plant Physiol., 1996, vol. 149, pp. 439-443.
Knight, R., Trewavas, A.J., and Knight, M.R., Cold Calcium Signaling in Arabidopsis Involves Two Cellular Pools and a Charge in Calcium Signature after Acclimation, Plant Cell, 1996, vol. 8, pp. 489-503.
Sheen, J., Mutational Analysis of a Protein Phosphatase 2C Involved in Abscisic Acid Signal Transduction in Higher Plants, Proc. Natl. Acad. Sci. USA, 1998, vol. 95, pp. 975-980.
Thion, L., Mazars, C., Nacry, P., and Bouchez, D., Plasma Membrane Depolarization-Activated Calcium Channels, Stimulated by Microtubule-Depolymerizing Drugs in Wild-Type Arabidopsis thaliana Protoplasts, Display Constitutively Large Activities and a Longer Half-Life in Ton 2 Mutant Cells Affected in the Organization of Cortical Microtubules, Plant J., 1998, vol. 13, pp. 603-610.
Pihakaski-Maunsbach, K. and Puhakainen, T., Effect of Cold Exposure on Cortical Microtubules of Rye (Secale cereale) as Observed by Immunocytochemistry, Physiol. Plant., 1995, vol. 93, pp. 563-571.
Khokhlova, L.P., Palikh, E., Olinevich, O.V., and Tarakanova, N.Yu., The Effect of Cytochalasin B and Colchicine on Water Metabolism during Cold Hardening and Freezing-Thawing at Different Regimes, Tsitologiya, 1997, vol. 39, pp. 294-304.
Aström, H., Virtanen, I., and Raudaskoski, M., Cold-Stability in the Pollen Tube Cytoskeleton, Protoplasma, 1991, vol. 160, pp. 99-111.
Baluška, F., Parker, J.S., and Barlow, P.W., The Microtubular Cytoskeleton in Cells of Cold-Treated Roots of Maize (Zea mays) Shows Tissue-Specific Responses, Protoplasma, 1993, vol. 172, pp. 84-96.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Olinevich, O.V., Khokhlova, L.P. Cytoskeleton-Dependent Changes in the Structural Organization of Reticuloplasmins in Triticum aestivum Cells during Cold Acclimation and Treatment with Abscisic Acid. Russian Journal of Plant Physiology 49, 196–203 (2002). https://doi.org/10.1023/A:1014845405575
Issue Date:
DOI: https://doi.org/10.1023/A:1014845405575