Abstract
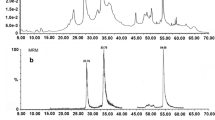
This study aimed to evaluate the antifungal effect of SC319 sorghum phenolic extract (SPE) on the Aspergillus, Fusarium, Penicillium, Stenocarpella, Colletotrichum, and Macrophomina genera. SPE was extracted by 20% ethanol and used in four assays: (1) against Fusarium verticillioides in solid (PDA) and liquid (PD) potato dextrose media; (2) Minimum Inhibitory Concentration (MIC) assay with 16 fungi isolates; (3) Conidial Germination Rate (CGR) with 14 fungi isolates and (4) Growth Curve (GC) with 11 fungi isolates. There was no reduction in the mycelial growth (colony diameter and dry weight) and in the number of Fusarium verticillioides spores in assay 1 (PDA and PD). The colony's dry weight was almost six times higher in the presence than in the absence of SPE. All SPE samples presented MIC (assay 1) above the maximum concentration tested (5000 µg.mL−1) for the 16 isolates. Also, there was no inhibitory effect of SPE on conidia germination rate (CGR). Oppositely, in GC assay, the control had a higher CFU count than the samples with SPE in 24 h. This result suggests that SPE can delay the fungal growth in the first hours of incubation, which is an important finding that may help reduce the severity of fungal diseases in plants. However, further studies are needed to confirm these results, including sorghum genotypes with different profiles of phenolic compounds. Although the SC319 SPE was not effective as an antifungal agent, it may have potential as a growth promoter of beneficial fungi in the food and pharmaceutical industries.



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Campos EV, Proença PL, Oliveira JL, Bakshi M, Abhilash PC, Fraceto LF (2019) Use of botanical insecticides for sustainable agriculture: future perspectives. Ecol Ind 105:483–495. https://doi.org/10.1016/j.ecolind.2018.04.038
Zambolim L, Juliatti FC, Guerra W (2021) How to cope with the vulnerability of site specific fungicides on the control of Asian soybean rust. Int J Res Agron 4(1):14–25. https://doi.org/10.33545/2618060X.2021.v4.i1a.4
Scorzoni L, Sangalli-Leite F, Singulani JL, Silva ACAP, Costa-Orlandi CB, Fusco-Almeida AM, Mendes-Giannini MJS (2016) Searching new antifungals: the use of in vitro and in vivo methods for evaluation of natural compounds. J Microbiol Methods 123:68–78. https://doi.org/10.1016/j.mimet.2016.02.005
Silva NCC, FernandesJúnior A (2010) Biological properties of medicinal plants: a review of their antimicrobial activity. J Venomous Anim Toxins 16(3):402–413. https://doi.org/10.1590/S1678-91992010000300006
Haminiuk WIC, Maciel GM, Plata-Oviedo MSV, Peralta RM (2012) Phenolic compounds in fruits: an overviewm. Int J Food Sci Technol 47(10):2023–2044. https://doi.org/10.1111/j.1365-2621.2012.03067.x
Serrano J, Puupponen-Pimiã R, Dauer A, Aura AM, Saura-Calixto F (2009) Tanins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 53:310–329. https://doi.org/10.1002/mnfr.200900039
Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
Aguilera-Carbo C, Augur LA, Prado-Barragan E, Favela-Torres CN (2008) Aguilar microbial production of ellagic acid and biodegradation of ellagitannins. Appl Microbiol Biotechnol 78:189–199. https://doi.org/10.1007/s00253-007-1276-2
Akhtar S, Ismail T, Fraternale D, Sestili P (2015) Pomegranate peel and peel extracts: chemistry and food features. Food Chem 174:417–425. https://doi.org/10.1016/j.foodchem.2014.11.035
Elsherbiny EA, Amin BH, Baka ZA (2016) Efficiency of pomegranate (Punicagranatum L.) peels extract as a high potential natural tool towards Fusarium dry rot on potato tubers. Postharvest Biol Technol 111:256–263. https://doi.org/10.1016/j.postharvbio.2015.09.019
Kharchoufi S, Licciardello F, Siracusa L, Muratore G, Hamdi M, Restuccia C (2018) Antimicrobial and antioxidant features of “Gabsi” pomegranate peel extracts. Ind Crops Prod 111:345–352. https://doi.org/10.1016/j.indcrop.2017.10.037
Awika JM, Rooney LW (2004) Sorghum phytochemical and their potential impact on human health. Phytochemistry 65:1199–1221
Silva TL, Lacerda UV, Matta SLP, Queiroz VAV, Stringheta PC, Martino HSD, Barros FA (2020) Evaluation of the efficacy of toasted white and tannin sorghum flours to improve oxidative stress and lipid profile in vivo. J Food Sci 85(7):2236–2244. https://doi.org/10.1111/1750-3841.15301
Martinez ODM, Theodoro JMV, Grancieri M, Toledo RCL, Queiroz VAV, Barros FAR, Martino HSD (2021) Dry heated whole sorghum flour (BRS 305) with high tannin and resistant starch improves glucose metabolism, modulates adiposity, and reduces liver steatosis and lipogenesis in Wistar rats fed with a high-fat high-fructose diet. J Cereal Sci 99:Article 103201. https://doi.org/10.1016/j.jcs.2021.103201
Moraes EA, Marinelli RS, Lenquiste SA, Steel CJ, Menezes CB, Queiroz VAV, MarósticaJúnior MR (2015) Sorghum flour fractions: correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem 180:116–123. https://doi.org/10.1016/j.foodchem.2015.02.023
Kil HY, Seong ES, Ghimire BK, Chung I, Kwon SS, Goh EJ, Heo KH, Kim MJ, Lim JD, Lee D, Yu CY (2009) Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem 115(4):1234–1239. https://doi.org/10.1016/j.foodchem.2009.01.032
Oliveira KG, Queiroz VAV, Carlos LA, Cardoso LM, Pinheirosant’Ana HM, Anunciação PC, Menezes CB, Silva EC, Barros FAR (2017) Effect of the storage time and temperature on phenolic compounds of sorghum grain and flour. Food Chem 216:390–398. https://doi.org/10.1016/j.foodchem.2016.08.047
Anunciação PC, Cardoso LM, Gomes JVP, Della Lucia CM, Carvalho CWP, Galdeano MC, Queiroz VAV, Alfenas RCG, Martino HSD, Pinheiro-Sant’Ana HM (2017) Comparing sorghum and wheat whole grain breakfast cereals: sensorial acceptance and bioactive compound content. Food Chem 221:984–989
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenol and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–177
Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L (2003) Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem 51(23):6657–6662. https://doi.org/10.1021/jf034790i
Castellani A (1939) Viability of some pathogenic fungi in distilled water. J Trop Med Hyg 24:270–276
Santos DA, Hamdan JS (2005) Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J Clin Microbiol 43(4):1917–1920. https://doi.org/10.1128/jcm.43.4.1917-1920.2005
Clinical and Laboratory Standards Institute (2017) Performance standards for antifungal susceptibility testing of yeasts, 1st edn. Clinical and Laboratory Standards Institute. CLSI supplement M60
Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, de Hoog GS, Meis JF (2015) Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother 70(4):1068–1071
Borman AM, Fraser M, Palmer MD, Szekely A, Houldsworth M, Patterson Z, Johnson EM (2017) MIC distributions and evaluation of fungicidal activity for amphotericin B, itraconazole, voriconazole, posaconazole and caspofungin and 20 species of pathogenic filamentous fungi determined using the CLSI broth microdilution method. J Fungi 3(2):27
Liu T, Zhang Q, Wang L, Yu L, Leng W, Yang J, Chen L, Peng P, Ma L, Dong J, Xu X, Xue Y, Zhu Y, Zhang W, Yang L, Li W, Sun L, Wan Z, Ding G, Yu F, Tu K, Qian Z, Li R, Shen Y, Li Y, Jin Q (2007) The use of global transcriptional analysis to reveal the biological and cellular events involved in distinct development phases of Trichophyton rubrum conidial germination. BMC Genomics 8:Article 100. https://doi.org/10.1186/1471-2164-8-100
Ferreira DF (2003) Programa SISVAR: sistema de análise de variância: versão 4.6 (Build 6.0). Universidade Federal de Lavras
AtaeiAzimi AA, DelnavazHashemloian B, Mansoorghanaei A (2007) Antifungal effects of water, alcoholic and phenolic extracts of seeds and leaves of Sorghum bicolor [L.] Moench on Fusarium solani and F. poae. J Med Plants 6:26–32
Javaid A, Naqvi SF, Shoaib A (2012) Antifungal activity of methanolic extracts of Sorghum halepense against Macrophomina phaseolina. Afr J Microbiol Res 6(28):5814–5818
Naeem Z, Jabeen K, Saeed MK, Iqbal S (2021) Phytochmical analysis and antifungal potential of Sorghum Halepense (L.) Pers. for the management of mycotoxigenic fungi. Pakistan J Weed Sci Res 27(4). https://doi.org/10.28941/pjwsr.v27i4.1008
Ratnavathi CV, Sashidhar RB (2007) Inhibitory effect of phenolics extracted from sorghum genotypes on Aspergillus parasiticus (NRRL 2999) growth and aflatoxin production. J Sci Food Agric 87(6):1140–1148. https://doi.org/10.1002/jsfa.2827
Funnell-Harris DL, O’neill PM, Sattler SE, Gries T, Berhow MA, Pedersen JF (2017) Response of sorghum stalk pathogens to brown midrib plants and soluble phenolic extracts from near isogenic lines. Eur J Plant Pathol 148(4):941–953. https://doi.org/10.1007/s10658-017-1148-2
Funnell-Harris DL, Sattler SE, Pedersen JF (2014) Response of Fusarium thapsinum to sorghum brown midrib lines and to phenolic metabolites. Plant Dis 98:1300–1308
Schöneberg T, Kibler K, Sulyok M, Musa T, Bucheli DT, Mascher F, Bertossa M, Voegele RT, Vogelgsang S (2018) Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit Contam Part A 35(12):2455–2470. https://doi.org/10.1080/19440049.2018.1538570
Gordana SC, Jasna MC, Sonja MD, Tumbas VT, Markov SL, Dragoljub DC (2007) Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L. Subsp. kitaibelii extracts. Int J Mol Sci 8(10):1013–1027
Gauthier L, Bonnin-Verdal MN, Marchegay G, Pinson-Gadais L, Ducos C, Richard-Forget F, Atanasova-Pénichon V (2016) Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: new insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereal. Int J Food Microbiol 221:61–68
Dzhavakhiya V, Shcherbakova L, Semina Y, Zhemchuzhina N, Campbell B (2012) Chemosensitization of plant Pathogenic fungi to agricultural fungicides. Front Microbiol 3:87
Paul S, Dubey RC, Maheswari DK, Kang SC (2011) Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 22:725–731
Da Silva Bomfim N, Nakassugi LP, Pinheiro Oliveira JF, Kohiyama CY, GaleraniMossini SA, BotiãoNerilo RGS, Mallmann CA, Abreu Filho BA, Machinski M (2015) Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.). Food Chem 166:330–336
Acknowledgements
The authors acknowledge the Empresa Brasileira de Pesquisa Agropecuária – Embrapa, Brazil (grant number SEG 20.18.03.006.00.00) and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG, Brazil (grant number APQ-00657-18) for the financial support and the Conselho Nacional de Desenvolvimento Científico e Tecnológico– CNPq, Brazil, for for the granting of the PhD student scholarship (grant number 142352/2016-0).
Author information
Authors and Affiliations
Contributions
Renata R. P. da Conceição: Conceptualization, Methodology, Investigation, Data curation, Resources, Writing – original draft. Valéria A. V. Queiroz: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision. Maria Lúcia F. Simeone: Conceptualization, Methodology, Writing – review & editing, Supervision. Dagma D. da Silva: Methodology, Writing – review & editing, Project administration, funding acquisition. Maria Aparecida de R. Stoianoff: Conceptualization, Methodology, Writing – review & editing, Supervision, and José Edson F. Figueiredo: Writing – review & editing.
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Jerri Zilli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Conceição, R.R.P., Queiroz, V.A.V., Simeone, M.L.F. et al. Does sorghum phenolic extract have antifungal effect?. Braz J Microbiol 55, 1829–1839 (2024). https://doi.org/10.1007/s42770-024-01327-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01327-9