Abstract
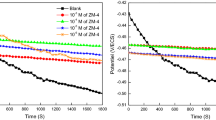
In the present study, our main objective was to assess the effect of a synthetic organic inhibitor namely 2-((1-benzyl-1H-1,2,3-triazol-4-yl) methyl) benzo[d]isothiazol-3(2H)-one 1,1-dioxide, commonly abbreviated as "BTMS," on the corrosion behavior of E24 steel when subjected to a 1 M HCl solution. To achieve this goal, we utilized the open circuit potential (OCP), potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS). Also, we performed surface morphologies through a combined approach involving scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX). The PDP investigation revealed that BTMS operated as a mixed-type inhibitor. It had a maximum efficiency of 92.96 at 1 mM, the PDP measurements confirm the results obtained by the EIS data. The influence of temperature on the corrosion behavior with the addition of BTMS was examined in the temperature range of 293–323 K. The interaction and binding of our synthesized inhibitor onto the surface of E24 steel conformed to the Langmuir isotherm model, with adsorption defined as both physisorption and chemisorption occurs, although physisorption predominates. The SEM/EDX examination definitively demonstrated the establishment of a barrier of protection and the corrosion-inhibiting capabilities of BTMS. In addition, computational methods based on detailed electronic-scale (density functional theory (DFT)) and atomic-scale simulations investigations based on MC (Monte Carlo), and MD (Molecular dynamic) approaches agree well with the effectiveness of inhibition performance revealed by the experimental results. The findings of this study have important significance for researchers seeking to develop more effective synthetic organic inhibitors to prevent metal corrosion.






















Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Azgaou K, Damej M, El Hajjaji S, Sebbar NK, Elmsellem H, El Ibrahimi B, Benmessaoud M (2022) Synthesis and characterization of N-(2-aminophenyl)-2-(5-methyl-1H-pyrazol-3-yl) acetamide (AMPA) and its use as a corrosion inhibitor for C38 steel in 1 M HCl. Experimental and theoretical study. J Mol Struct 1266:133451
El Hamdouni Y, Bouhlal F, Kouri H, Chellouli M, Benmessaoud M, Dahrouch A, Labjar N, El Hajjaji S (2020) Use of omeprazole as inhibitor for C38 steel corrosion in 1.0 M H3PO4 medium. J Fail Anal Prev 20:563–571
Bouhlal F, Mazkour A, Labjar H, Benmessaoud M, Serghini-Idrissi M, El Mahi M, El Hajjaji S, Labjar N (2020) Combination effect of hydro-alcoholic extract of spent coffee grounds (HECG) and potassium Iodide (KI) on the C38 steel corrosion inhibition in 1M HCl medium: experimental design by response surface methodology. Chem Data Collect 29:100499
Al Maofari A, Ezznaydy G, Idouli Y, Guédira F, Zaydoun S, Labjar N, El S (2014) Inhibitive action of 3, 4’-bi-1, 2, 4-Triazole on the corrosion of copper in NaCl 3% solution. J Mater Environ Sci 5:2081
Thakur A, Sharma PK, Kumar A (2022) Economics and commercialization of carbon allotropes nanostructured corrosion inhibitors. Carbon Allotropes Nanostruct Anti-Corrosive Mater 383
Abouchane M, Hsissou R, Molhi A, Damej M, Tassaoui K, Berisha A, Chraka A, Benmessaoud M (2023) Exploratory experiments supported by modeling approaches for TGEEA new epoxy resin as a contemporary anti-corrosion material for C38 steel in 1.0 M HCl. J Fail Anal Prev 23:1765–1781
Akounach Z, Al Maofari A, El Yadini A, Douche S, Benmessaoud M, Ouaki B, Damej M, Hajjaji SE (2018) Inhibition of mild steel corrosion in 1.0 M HCl by water, hexane and ethanol extracts of pimpinella anisum plant. Anal Bioanal Electrochem 10:1506
Emori W, Louis H, Okonkwo PC, Njoku DI, Edet HO, Okafor PC, Cheng C-R (2023) Dispersive adsorption and anticorrosion properties of natural capsaicin on Q235 steel in mixed H2SO4 and NaCl environment: characterization, experimental and theoretical studies. Sustain Chem Pharm 32:101042
Damej M, Molhi A, Lgaz H, Hsissou R, Aslam J, Benmessaoud M, Rezki N, Lee H, Lee D (2023) Performance and interaction mechanism of a new highly efficient benzimidazole-based epoxy resin for corrosion inhibition of carbon steel in HCl: a study based on experimental and first-principles DFTB simulations. J Mol Struct 1273:134232
Damej M, Hsissou R, Berisha A, Azgaou K, Sadiku M, Benmessaoud M, Labjar N (2022) New epoxy resin as a corrosion inhibitor for the protection of carbon steel C38 in 1M HCl. experimental and theoretical studies (DFT, MC, and MD). J Mol Struct 1254:132425
Damej M, Molhi A, Tassaoui K, El Ibrahimi B, Akounach Z, Addi AA, El Hajjaji S, Benmessaoud M (2022) Experimental and theoretical study to understand the adsorption process of p-Anisidine and 4-Nitroaniline for the dissolution of C38 carbon steel in 1M HCl. ChemistrySelect 7:e202103192
Tassaoui K, Al-Shami A, Damej M, Molhi A, Mounkachi O, Benmessaoud M (2023) Contribution to the corrosion inhibitors of copper-nickel (Cu-30Ni) in 3% NaCl solution by two new molecules of triazole: electrochemical and theoretical studies. Journal of Molecular Structure 1291:135836
Verma C, Hussain CM, Quraishi M, Alfantazi A (2023) Green surfactants for corrosion control: design, performance and applications. Adv Coll Interface Sci 311:102822
Akroujai E, Chetioui S, Benzbiria N, Barrahi A, Chraka A, Djedouani A, Chtita S, Dikici B, Warad, Bellaouchou A (2023) Electrochemical, surface analysis, computational and anticorrosive studies of novel naphthalene derivative on carbon steel surface, Int J Corros Scale Inhib 12
Manssouri M, El Ouadi Y, Chraka A, Khaddor M, Znini M, Majidi L (2021) Aqueous extracts of Aaronsohnia pubescens subsp. pubescens aerial parts as Green Corrosion Inhibitor for Mild Steel in hydrochloric acid solution. J Turk Chem Soc Sect A Chem 8:953–968
Yang X, Lu X, Zhou Y, Xie Y, Yang J, Wang F (2023) Formation of protective conversion coating on Mg surface by inorganic inhibitor. Corros Sci 215:111044
Belhadi M, Oubahou M, Hammoudan I, Chraka A, Chafi M, Tighadouini S (2023) A comprehensive assessment of carbon steel corrosion inhibition by 1,10-phenanthroline in the acidic environment: insights from experimental and computational studies, Environmental Science and Pollution Research, pp 1–18
Al-Amiery AA, Isahak WNRW, Al-Azzawi WK (2023) Corrosion inhibitors: natural and synthetic organic inhibitors. Lubricants 11:174
Al-Amiery AA, Al-Azzawi WK (2023) Organic synthesized inhibitors for corrosion protection of carbon steel: a comprehensive review. J Bio-and Tribo-Corros 9:74
Damej M, Abouchane M, Doubi M, Erramli H, Benmessaoud M, Hajjaji N (2022) Electrodeposition and characterization of poly 3-Amino-1, 2, 4-Triazole-5-Thiol Films on brass electrode in 0.1 M methanol. Coatings 12:1784
Mishra A, Verma C, Lgaz H, Srivastava V, Quraishi M, Ebenso EE (2018) Synthesis, characterization and corrosion inhibition studies of N-phenyl-benzamides on the acidic corrosion of mild steel: experimental and computational studies. J Mol Liq 251:317–332
Verma C, Verma DK, Ebenso EE, Quraishi MA (2018) Sulfur and phosphorus heteroatom-containing compounds as corrosion inhibitors: an overview. Heteroat Chem 29:e21437
Singh AK, Thakur S, Pani B, Ebenso EE, Quraishi MA, Pandey AK (2018) 2-Hydroxy-N′-((Thiophene-2-yl) methylene) benzohydrazide: ultrasound-assisted synthesis and corrosion inhibition study. ACS Omega 3:4695–4705
Lakbaibi Z, Damej M, Molhi A, Benmessaoud M, Tighadouini S, Jaafar A, Benabbouha T, Ansari A, Driouich A, Tabyaoui M (2022) Evaluation of inhibitive corrosion potential of symmetrical hydrazine derivatives containing nitrophenyl moiety in 1M HCl for C38 steel: experimental and theoretical studies, Heliyon. 8:e09087
Chraka A, Raissouni I, Seddik NB, Khayar S, Mansour AI, Tazi S, Chaouket F, Bouchta D (2020) Identification of potential green inhibitors extracted from Thymbra capitata (L.) Cav. for the corrosion of Brass in 3% NaCl solution: experimental, SEM–EDX analysis, DFT computation and Monte Carlo simulation studies. J Bio-and Tribo-Corros 6:1–19
Chraka A, Raissouni I, Benseddik N, Khayar S, Mansour AI, Belcadi H, Chaouket F, Bouchta D (2020) Aging time effect of Ammi visnaga (L.) lam essential oil on the chemical composition and corrosion inhibition of brass in 3% NaCl medium. Exp Theor Stud Mater Today: Proc 22:83–88
Brahim El I, Lei G (2020) Azole-Based Compounds as Corrosion Inhibitors for Metallic Materials in Azoles (Aleksey, K., ed), IntechOpen, Rijeka, pp Ch. 5
Marinescu M (2019) Recent advances in the use of benzimidazoles as corrosion inhibitors. BMC Chem 13:1–21
Verma C, Thakur A, Ganjoo R, Sharma S, Assad H, Kumar A, Quraishi M, Alfantazi A (2023) Coordination bonding and corrosion inhibition potential of nitrogen-rich heterocycles: azoles and triazines as specific examples. Coord Chem Rev 488:215177
Resende GO, Teixeira SF, Figueiredo IF, Godoy AA, Lougon DJF, Cotrim BA, Souza FCd (2019) Synthesis of 1,2,3-Triazole Derivatives and Its Evaluation as Corrosion Inhibitors for Carbon Steel. International Journal of Electrochemistry 2019:6759478
Rahmani H, Alaoui KI, Emran K, El Hallaoui A, Taleb M, El Hajji S, Labriti B, Ech-Chihbi E, Hammouti B, El-Hajjaji F (2019) Experimental and DFT investigation on the corrosion inhibition of mild steel by 1, 2, 3-triazole regioisomers in 1M hydrochloric acid solution. Int J Electrochem Sci 14:985–998
Akounach Z, Al Maofari A, Damej M, El Hajjaji S, Berisha A, Mehmeti V, Labjar N, Bamaarouf M, Benmessaoud M (2022) Contribution to the corrosion inhibition of aluminum in 1 M HCl by Pimpinella Anisum extract. Experimental and theoretical studies (DFT, MC, and MD). Int J Corros Scale Inhib 11:402–424
Chraka A, Seddik NB, Raissouni I, Kassout J, Choukairi M, Ezzaki M, Zaraali O, Belcadi H, Janoub F, Mansour AI (2023) Electrochemical explorations, SEM/EDX analysis, and quantum mechanics/molecular simulations studies of sustainable corrosion inhibitors on the Cu-Zn alloy in 3% NaCl solution. J Mol Liq 387:122715
El Mahmoudi A, El Masaoudi H, Tachallait H, Talha A, Arshad S, Benhida R, Jaber B, Benaissa M, Bougrin K (2022) Selective silver (I)-catalyzed four-component gram-scale synthesis of novel 1, 4-disubstituted 1, 2, 3-triazole-sulfonamides under heterogeneous catalysis and microwave irradiation in water. Results in Chemistry 4:100552
Al-Sharabi H, Bouhlal F, Bouiti K, Labjar N, Al Zalaei E, Dahrouch A, Benabdellah G, El Mahi M, Benmessaoud B, Lotfi E (2022) Electrochemical and thermodynamic evaluation on corrosion inhibition of C38 steel in 1 M HCl by Rumex ethanolic extract. Int J Corros Scale Inhib 11:382–401
Al Maofaria A, Doucha S, Benmessaouda M, Hajjaji S, Mosaddak M, Ouaki B (2021) Inhibition study of various extracts of tribulus terrestris plant on the corrosion of mild steel in a 1.0 M HCl solution. Port Electrochim Acta 39:21–35
Belcadi H, Chraka A, El Amrani S, Raissouni I, Moukhles A, Zantar S, Toukour L, Mansour AI (2023) Investigation and Valorization of the Moroccan Salvia Officinalis L. Essential Oil: Phytochemistry, Potential in Corrosion Inhibition, Antibacterial Activity, and Theoretical Modeling. J Bio-and Tribo-Corros 9:50
Baldauf M, Seifert A, Förstner J, Majewski D, Raschendorfer M, Reinhardt T (2011) Operational convective-scale numerical weather prediction with the COSMO model: description and sensitivities. Mon Weather Rev 139:3887–3905
Akkermans RL, Spenley NA, Robertson SH (2021) COMPASS III: automated fitting workflows and extension to ionic liquids. Mol Simul 47:540–551
Rosales-Pelaez P, Sanchez-Burgos I, Valeriani C, Vega C, Sanz E (2020) Seeding approach to nucleation in the N V T ensemble: the case of bubble cavitation in overstretched Lennard Jones fluids. Phys Rev E 101:022611
Ajebli S, Kaichouh G, Khachani M, Babas H, El Karbane M, Warad I, Safi Z, Berisha A, Mehmeti V, Guenbour A (2022) The adsorption of Tenofovir in aqueous solution on activated carbon produced from maize cobs: insights from experimental, molecular dynamics simulation, and DFT calculations. Chem Phys Lett 801:139676
Otaifah YN, Hussein K, Benmessaoud M, El Hajjaji S (2017) Study of the inhibitory effect of the Jasminum sambac extract on the corrosion of dental amalgam in saliva media. J Int Dent Med Res 10:222
El-Asri A, Rguiti M, Jmiai A, Oukhrib R, Bourzi H, Lin Y, El Issami S (2023) Carissa macrocarpa extract (ECM) as a new efficient and ecologically friendly corrosion inhibitor for copper in nitric acid: Experimental and theoretical approach. J Taiwan Inst Chem Eng 142:104633
Ouass A, Galai M, Ouakki M, Ech-Chihbi E, Kadiri L, Hsissou R, Essaadaoui Y, Berisha A, Cherkaoui M, Lebkiri A (2021) Poly (sodium acrylate) and Poly (acrylic acid sodium) as an eco-friendly corrosion inhibitor of mild steel in normal hydrochloric acid: experimental, spectroscopic and theoretical approach. J Appl Electrochem 51:1009–1032
Tassaoui K, Damej M, Molhi A, Berisha A, Errili M, Ksama S, Mehmeti V, El Hajjaji S, Benmessaoud M (2022) Contribution to the corrosion inhibition of Cu–30Ni copper–nickel alloy by 3-amino-1, 2, 4-triazole-5-thiol (ATT) in 3% NaCl solution. Experimental and theoretical study (DFT, MC and MD). Int J Corros Scale Inhib 11:221–244
Abdelwedoud BO, Damej M, Tassaoui K, Berisha A, Tachallait H, Bougrin K, Mehmeti V, Benmessaoud M (2022) Inhibition effect of N-propargyl saccharin as corrosion inhibitor of C38 steel in 1 M HCl, experimental and theoretical study. J Mol Liq 354:118784
Lebkiri I, Abbou B, Hsissou R, Safi Z, Sadiku M, Berisha A, El Amri A, Essaadaoui Y, Kadiri L, Lebkiri A (2023) Investigation of the anionic polyacrylamide as a potential adsorbent of crystal violet dye from aqueous solution: Equilibrium, kinetic, thermodynamic, DFT, MC and MD approaches. J Mol Liq 372:121220
Haldhar R, Prasad D, Saxena A, Kaur A (2018) Corrosion resistance of mild steel in 0.5 MH 2 SO 4 solution by plant extract of Alkana tinctoria: experimental and theoretical studies. Eur Phys J Plus 133:1–18
Jafari H, Ameri E, Rezaeivala M, Berisha A (2022) Experimental and theoretical studies on protecting steel against 0.5 M H2SO4 corrosion by new schiff base. J. Indian Chem. Soc 99:100665
Abouchane M, Hsissou R, Chraka A, Molhi A, Damej M, Tassaoui K, Berisha A, Seydou M, Elemine BO, Benmessaoud M (2024) Synthesis and characterization of new macromolecular epoxy resin as an effective corrosion inhibitor for C38 steel in 1 M HCl medium: electrochemical insights, surface morphological and computational approaches. Journal of Bio-and Tribo-Corrosion 10:21
Babas H, Khachani M, Warad I, Ajebli S, Guessous A, Guenbour A, Safi Z, Berisha A, Bellaouchou A, Abdelkader Z (2022) Sofosbuvir adsorption onto activated carbon derived from argan shell residue: optimization, kinetic, thermodynamic and theoretical approaches. J Mol Liq 356:119019
Berrissoul A, Ouarhach A, Benhiba F, Romane A, Zarrouk A, Guenbour A, Dikici B, Dafali A (2020) Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution. Experimental and theoretical approach. J Mol Liq 313:113493
Zhang K, Yang W, Xu B, Chen Y, Yin X, Liu Y, Zuo H (2018) Inhibitory effect of konjac glucomanan on pitting corrosion of AA5052 aluminium alloy in NaCl solution. J Colloid Interface Sci 517:52–60
Ganjoo R, Sharma S, Thakur A, Assad H, Sharma PK, Dagdag O, Berisha A, Seydou M, Ebenso EE, Kumar A (2022) Experimental and theoretical study of Sodium Cocoyl Glycinate as corrosion inhibitor for mild steel in hydrochloric acid medium. J Mol Liq 364:119988
Frey R, Werder R, Günthard HH (1970) Far infrared matrix spectra of iron chlorides Fe2Cl6, FeCl3, Fe2Cl4, and FeCl2. J Mol Spectrosc 35:260–284
Thibeau RJ, Brown CW, Heidersbach RH (1978) Raman spectra of possible corrosion products of iron. Appl Spectrosc 32:532–535
Chraka A, Raissouni I, Kassout J, Ezzaki M, Ben Seddik N, Janoub F, Manssouri M, Belcadi H, Ibn Mansour A, Bouchta D (2023) Understanding the synergistic inhibition effect of hydrosol extract derivatives as eco-friendly anti-corrosive for copper alloy: GC–MS Identification, An Electrochemical, surface morphology and computational modeling. Journal of Molecular Liquids 392:123507
Chraka A, Raissouni I, Seddik, NB, Khayar S, El Amrani S, El Hadri M, Chaouket F, Bouchta D (2020) Croweacin and Ammi visnaga (L.) lam essential oil derivatives as green corrosion inhibitors for brass in 3% NaCl medium: quantum mechanics investigation and molecular dynamics simulation approaches. Mediterr J Chem 10:378
Ouaket A, Chraka A, Raissouni I, El Amrani MA, Berrada M, Knouzi N (2022) Synthesis, spectroscopic (13C/1H-NMR, FT-IR) investigations, quantum chemical modelling (FMO, MEP, NBO analysis), and antioxidant activity of the bis-benzimidazole molecule. J Mol Struct 1259:132729
Kumar H, Dhanda T (2021) Cyclohexylamine an effective corrosion inhibitor for mild steel in 0.1 N H2SO4: experimental and theoretical (molecular dynamics simulation and FMO) study. J Mol Liq 327;114847
Eliboev I, Berdimurodov E, Yakhshinorov K, Abdisattarov J, Dagdag O, Berisha A, Nik WW, Kholikov A, Akbarov K (2023) Supramolecular corrosion protection: eco-friendly synthesis and efficacy of a β-cyclodextrin/o-phenylenediamine complex. J Taiwan Inst Chem Eng 147:104944
Hsissou R, Benhiba F, Abbout S, Dagdag O, Benkhaya S, Berisha A, Erramli H, Elharfi A (2020) Trifunctional epoxy polymer as corrosion inhibition material for carbon steel in 1.0 M HCl: MD simulations, DFT and complexation computations. Inorg Chem Commun 115:107858
Rahmani R, Boukabcha N, Chouaih A, Hamzaoui F, Goumri-Said S (2018) On the molecular structure, vibrational spectra, HOMO-LUMO, molecular electrostatic potential, UV–Vis, first order hyperpolarizability, and thermodynamic investigations of 3-(4-chlorophenyl)-1-(1yridine-3-yl) prop-2-en-1-one by quantum chemistry calculations. J Mol Struct 1155:484–495
Jafari H, Ameri E, Rezaeivala M, Berisha A, Vakili MH (2022) Comparison the anticorrosion behavior of three symmetrical Schiff-base ligands: experimental and theoretical studies. J Appl Electrochem 52:1803–1818
Lgaz H, Salghi R, Masroor S, Kim S-H, Kwon C, Kim SY, Yang Y-J, Chung I-M (2020) Assessing corrosion inhibition characteristics of hydrazone derivatives on mild steel in HCl: insights from electronic-scale DFT and atomic-scale molecular dynamics. J Mol Liq 308:112998
Huang Q, Lu W, Zhu J, Liu J, Hu P, Gan B, Qiu L (2024) Experimental and theoretical investigation of phenyl disulphide as an effective inhibitor for copper in sodium chloride medium. Int J Electrochem Sci 19:100435
Zheng T, Wang L, Liu J, Wang J, Jia G (2021) The corrosion inhibition effect of sodium silicate and Triton X-100 on 2024–T3 aluminum alloy in NaOH medium: experimental and theoretical research. Colloids Surf, A 610:125723
Abd El-Lateef HM, Shaaban S, Shalabi K, Khalaf MM (2022) Novel organoselenium-based N-mealanilic acids as efficacious corrosion inhibitors for 6061 aluminum alloy in molar HCl: in-silico modeling, electrochemical, and surface morphology studies. J Taiwan Inst Chem Eng 133:104258
Radi M, Melian R, Galai M, Dkhirche N, Makha M, Verma C, Fernandez C, EbnTouhami M (2021) Pumpkin seeds as an eco-friendly corrosion inhibitor for 7075-T6 alloy in 3.5% NaCl solution: electrochemical, surface and computational studies. J Mol Liq 337:116547
Kokalj A (2012) On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem Phys 393:1–12
Özbakır Işın D, Karakuş N, Lgaz H, Kaya S, Chung I-M (2020) Theoretical insights about inhibition efficiencies of some 8-Hydroxyqionoline derivatives against the corrosion of mild steel. Mol Simul 46:1398–1404
Sastri VS, Perumareddi JR (1997) Molecular Orbital Theoretical Studies of Some Organic Corrosion Inhibitors, Corrosion, p 53
El Faydy M, About H, Warad I, Kerroum Y, Berisha A, Podvorica F, Bentiss F, Kaichouh G, Lakhrissi B, Zarrouk A (2021) Insight into the corrosion inhibition of new bis-quinolin-8-ols derivatives as highly efficient inhibitors for C35E steel in 0.5 M H2SO4. J Mol Liq 342:117333
Berisha A (2021) Ab inito exploration of nanocars as potential corrosion inhibitors. Comput Theor Chem 1201:113258
Alahiane M, Oukhrib R, Berisha A, Albrimi YA, Akbour RA, Abou Oualid H, Bourzi H, Assabbane A, Nahlé A, Hamdani M (2021) Electrochemical, thermodynamic and molecular dynamics studies of some benzoic acid derivatives on the corrosion inhibition of 316 stainless steel in HCl solutions. J Mol Liq 328:115413
Ech-Chihbi E, Nahlé A, Salim R, Benhiba F, Moussaif A, El-Hajjaji F, Oudda H, Guenbour A, Taleb M, Warad I (2020) Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 1.0 M HCl solution. J Alloys Compd 844:155842
Rbaa M, Benhiba F, Galai M, Abousalem AS, Ouakki M, Lai C-H, Lakhrissi B, Jama C, Warad I, Touhami ME (2020) Synthesis and characterization of novel Cu (II) and Zn (II) complexes of 5-{[(2-Hydroxyethyl) sulfanyl] methyl}-8-hydroxyquinoline as effective acid corrosion inhibitor by experimental and computational testings. Chem Phys Lett 754:137771
Belghiti M, Echihi S, Dafali A, Karzazi Y, Bakasse M, Elalaoui-Elabdallaoui H, Olasunkanmi L, Ebenso E, Tabyaoui M (2019) Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some hydrazine derivatives in phosphoric acid on mild steel surface. Appl Surf Sci 491:707–722
Gholivand K, Sarmadi-Babaee L, Faraghi M, Badalkhani-Khamseh F, Fallah N (2022) Heteroatom-containing phosphoramides as carbon steel corrosion inhibitors: density functional theory and molecular dynamics simulations. Chem Phys Impact 5:100099
Chen X, Chen Y, Cui J, Li Y, Liang Y, Cao G (2021) Molecular dynamics simulation and DFT calculation of “green” scale and corrosion inhibitor. Comput Mater Sci 188:110229
Hsissou R, Abbout S, Seghiri R, Rehioui M, Berisha A, Erramli H, Assouag M, Elharfi A (2020) Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: computational studies (DFT, MC and MD simulations). J Market Res 9:2691–2703
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Miloud Errili: Writing-original draft, Validation, Experimental, Formal analysis, Visualization. Anas Chraka: Writing-review & editing, Software, Conceptualization (supporting). Mohamed Damej: Conceptualization, Writing–review & editing. Mohamed El Mahdi Ansar: Investigation, Resources. Najoua Labjar: Validation, Resources. Ayoub El mahmoudi: Validation, Conceptualization. Khalid Bougrin: Investigation, Formal analysis. Avni Berisha: Methodology, Resources, Writing-original draft, Software. Mohammed Benmessaoud: Supervision, Writing – review & editing, Investigation, Methodology, Conceptualization.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The study does not require ethical approval.
The study did not involve any animal or human data or tissue.
Consent for Publication
All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors declare they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Errili, M., Chraka, A., Damej, M. et al. An Experimental Investigation Linked Detailed-Level Computer Modeling on the Corrosion Inhibitory Activity of 2-((1-benzyl-1H-1,2,3-triazol-4-yl) methyl) benzo(d)isothiazol-3(2H)-one 1,1-dioxide on E24 Steel in a 1 M HCl Environment. Chemistry Africa (2024). https://doi.org/10.1007/s42250-024-00977-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42250-024-00977-4