Abstract
Gas operations generate large volumes of wastewater, necessitating efficient water management schemes. This study evaluates a forward osmosis (FO) pilot plant for volumes reduction of gas industry process water (PW). The osmotic pressure difference between seawater (40 g/L Total Dissolved Solids (TDS)) and low salinity (2 g/L TDS) PW is used for the osmotic concentration (OC). In the OC, PW volumes get reduced, while diluted draw solution (DS) is directly discharged, obviating the high-energy DS recovery step. A thin-film composite hollow fiber (HF) FO membrane was tested under FO mode using synthetic solutions to assess the performance on the OC unit. Subsequently, the pilot unit was subjected to PW feed for 48 h of continuous operation, primarily to evaluate water flux, reverse solute flux (RSF), and membrane fouling. The cleaning requirement to remove contaminants from the membrane surface was examined. The membrane achieved a water flux and RSF between 11.5 to 6.43 LMH and 38.57 to 9.45 mmol h−1 m−2, respectively at feed recovery rates between 60 and 90%. The membrane achieved a water flux of 10 LMH, which slightly decreased to 9.6 after 48 h of operation, mainly due to inorganic scaling. Lastly, cleaning with citric acid succeeded in recovering the initial water flux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The need for clean energy is increasing rapidly since the current world population is expected to grow by 29%, reaching 9.8 billion by 2050 [1]. The oil and gas (O&G) sector will continue to be a key component of worldwide energy sources; however, O&G operations are associated with the generation of significant wastewater volumes. The proper treatment and management of this wastewater is a critical challenge faced by the O&G industry [2, 3]. The most common management scheme for O&G effluents is based on deep-well injection, which is restrained by limited formation aquifer capacity and governmental regulations that prevent groundwater contamination [4, 5]. These challenges highlight the need for reliable and resilient processes that can treat O&G wastewater before disposal or reuse [6].
Research on using forward osmosis (FO) to treat industrial wastewater has been active for decades [2, 7,8,9]. The FO technology is an inherently low-pressure system since it is based on a naturally occurring phenomenon: osmosis, where a high concentration solution, draw solution (DS), can be used to transfer pure water molecules from a low concentration stream, or feed. This research has led to the paradigm that FO is characterized by low energy demand and a lower fouling tendency than high-pressure membrane technologies [10, 11]. A further downstream step is required to separate pure water from DS to recover water permeate from the FO process, allowing DS re-concentration and reuse. DS regeneration has typically been based on reverse osmosis (RO) systems, which require the application of high pressure or membrane distillation (MD) [12, 13]. Applying high pressure requires high energy input, making this two-step FO technology an energy-intensive process [14, 15]. Several studies have investigated the use of FO technology to treat O&G effluents [16,17,18,19]; however, the technology’s feasibility is hindered by the energy required to reconcentrate the DS [14, 20]. FO applications that obviate this step can be energetically favorable since the standalone FO membrane’s energy requirement is close to zero.
Single-stage FO technologies, such as osmotic concentration (OC), are potentially energy-efficient alternatives for wastewater treatment [20,21,22]. The concept of a standalone FO process is applicable when there is another application or use for the diluted DS stream, such as when a highly concentrated nutrient solution can be used as DS in fertilizer-drawn forward osmosis (FDFO), where the DS is diluted by the FO membrane and can then be used for irrigation [23,24,25]. Another example of the standalone FO process is the single-step osmotic dilution (OD) of highly saline seawater, which has been applied as a pretreatment for conventional RO [26]. The application’s partitioning energy was lower than classical membrane processes, such as RO [20]. Using FO as saline seawater OD before the RO system has a 16.6% lower specific energy consumption than conventional RO. A comparatively low energy demand (~ 0.24 kWh/m3) was estimated for the FDFO process in bench-scale studies [23].
The O&G effluent stream can be used as a feed solution (FS), while highly saline seawater or reject brine is employed as DS in the OC process. A more concentrated feed at a reduced volume, as well as a diluted DS stream, is generated from the membrane unit when water molecules permeate through the membrane barrier from the FS to the DS; therefore, the OC process is favorable for applications targeting effluent disposal volume minimization. The OC diluted DS stream can be discharged into the sea since this stream is just seawater at reduced concentrations, unlike the waste products from conventional two-step FO (Fig. 1) [21]. The OC can dilute high salinity waste brine generated from desalination facilities and reduce effluent volumes. OC has an energy consumption that is 81% lower than conventional RO systems operating at the same recovery rate and a similar feed quality [27]. Limited studies have exploited the OC process for O&G applications even though the research on FO has been active for decades [21, 27,28,29,30,31,32,33,34]. A standalone FO system was deployed to reclaim the drilling waste used to dilute the draw solution for drilling completion application [34]. Another study evaluated the feasibility of using a single-stage OC process for 50% O&G effluent volume reduction [27].
The technical feasibility of the OC process is primarily controlled by the achievable membrane flux, fouling severity, and cleaning requirements for fouling amelioration [35, 36]. These performance parameters are determined by several factors, such as feed water quality, DS osmotic pressure, FS and DS flowrates, imposed initial flux, and FO module properties [37,38,39].
A thin-film composite (TFC) FO membrane demonstrated a water flux of 17.5 LMH at a 50% volume reduction rate in a previous bench-scale study performed by our group. This study also revealed a relatively slight flux decline of 15% due to membrane fouling; however, the flux increased by 97% when the membrane was cleaned with sodium dodecyl sulfate (SDS) [17]. Hickenbottom et al. [34] determined that a cellulose triacetate (CTA) FO membrane used to reduce wastewater from drilling operations demonstrated a flux of 2–5 LMH at a recovery rate of up to 80%. The CTA membrane rejected all organic and inorganic constituents well with minimal irreversible membrane fouling [34, 40].
Previous studies have been based mainly on bench-scale investigations and conducted for relatively short periods of 2–6 h, even though the OC approach potential has been demonstrated by our group and other researchers [21, 27, 34]. The OC process must be investigated at a larger scale and for a relatively long operation period to bridge the gap between bench-scale and real-world implementation, which is vitally important to examine the reliability and stability under conditions comparable to those in the industry. The process performance might differ in long-term operations in terms of fouling severity and subsequent cleaning requirements [41]. FO membranes, such as TFC and CTA, are commercially available and have been tested for short-term experiments; however, their feasibility for large-scale OC technology installations has not been investigated yet to the best of our knowledge.
We implemented a pilot-scale OC technology to minimize industrial PW volumes. A commercially available TFC-HF FO membrane was subjected to synthetic FS and DS to assess the membrane’s basic water flux, recovery rate, and reverse solute flux (RSF) performance. These preliminary experiments also identified the most suitable operating conditions by targeting four different volume reduction rates (60–90%). The OC unit was then subjected to the PW feed to reduce the effluent volume by at least 75%. The pilot unit stability was rigorously evaluated for a 48-h operation period, where fouling propensity and cleaning requirements were assessed.
2 Materials and methods
2.1 OC pilot plant
A simple and flexible pilot unit with a feed capacity of 5 m3 was designed and constructed at the Pilot Hall of Qatar University to test industrial wastewater. This unit was used to evaluate the TFC membrane’s performance when subjected to the wastewater feed solution’s osmotic concentration. The pilot plant was composed of 5 m3 reservoirs, circulating pumps (Sterlitech), a buffer feed tank, one transfer pump, an overflow pump, 5 μm filters, and a membrane module (Aromatec, Singapore) (Fig. 2). Process parameter measurements, such as flow rate, pressure, temperature, and water salinity based on conductivity meters, were obtained from the built-in online sensors (Omega, UK). A real-time PID controller (cRIO 9035, NI, USA) was used to operate the pilot system, and the data was monitored and recorded via the LabVIEW user interface. The membrane water flux was measured based on the change in the weight of the buffer tank with time.
2.2 FO membranes
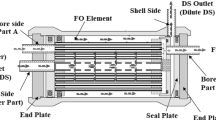
An Aromatec membrane, fabricated by Nanyang Technological University (NTU) and supplied by Aromatec Co., Ltd, Singapore, was used for the pilot study. This membrane is a TFC polyamide fabricated on a polyethersulfone support. The active layer is at the HF lumen, where the feed water is introduced, while the DS is pumped toward the shell side. The manufacturer’s module specifications are presented in Table 1.
The estimated permeability and salt rejection membrane properties of a virgin TFC membrane at different temperatures are summarized in Table 2. The feed solution was deionized water and 500 mg L−1 NaCl solution to obtain water and salt permeability values, A and B, respectively. Using RO mode, the system pressure was allowed to reach 2 bar for 60 s before collecting the permeate water.
2.3 Feed and draw solutions
Synthetic feed water (2000 mg L−1) was prepared by dissolving industrial grade sodium chloride salt (NaCl) with a purity of 99% in tap water that was pre-treated using a three-stage filter (5 microns, Altlas Filtri, Italy). The demonstration plant was also tested using onshore real PW, which is utilized during natural gas operations. Pre-treated membrane bioreactor (MBR) wastewater (5000 L) was obtained from the Qatargas industrial site. The effluent feed was analyzed for the organic and inorganic contaminants (Table 3). Hydrochloric acid (HCL) was added to the PW feed to maintain the pH within the TFC membrane’s tolerance range (7–8).
The draw solution used in this study was a synthetically prepared NaCl solution with a conductivity of 64,400 µS cm−1 to mimic the salinity of the seawater. Table 3 lists the detailed chemical composition of the feed and draw solutions.
2.4 Experimental procedures
The pilot testing program involved synthetic feed solutions and real wastewater feed trials. The TFC membrane was initially tested with the synthetic solutions for a short period of up to 4 h to evaluate the membrane properties and investigate the module’s capability of achieving high feed recoveries. Four different feed recoveries of 60%, 68%, 75%, and 90% were tested at a 75% DS dilution rate. The pilot plant was then subjected to a real PW stream for a long operation period of 50 h at a 75% feed recovery and 4 × dilution rate.
The Aromatec module was operated in countercurrent configuration in a recirculation mode, where the FS was applied to the bore-side inside the HF, and the DS was directed to flow over the shell side on the HF’s outer surface, or inside-out configuration (Fig. 3). The experiments were performed with a 20-L buffer tank placed on a digital balance. The FS and DS flowrates were maintained at a constant 1.5 L min−1; however, the DS flow was kept in once-through mode while the FS was set to recirculation mode by placing the concentrated FS stream, or outlet, in the FS tank, or buffer tank. The FS recirculation mode achieved higher recovery rates of up to 90% when the feed water in the buffer tank was concentrated due to the continuous addition of the concentrated outlet FS stream. An overflow pump was connected to the buffer tank to transfer the excess water when the tank level reached 20 L. Another transfer pump was installed to maintain the required feed recovery by adding feed water from the 5000 L tank. A higher feed recovery rate was obtained at a higher feed tank solution concentration. The percentage feed recovery rate, or volume reduction, of the feed and DS dilution are estimated as:
where \({V}_{\mathrm{initial}}\) and \({V}_{\mathrm{final}}\) are the FS volumes at the beginning of the experiment and when the required recovery rate was reached, respectively. \({Q}_{{DS}_{\mathrm{in}}}\) and \({Q}_{{DS}_{\mathrm{out}}}\) are the flowrates of the inlet and outlet DS streams in L min−1, respectively.
The change in buffer tank weight with time was recorded by the data acquisition system (LabVIEW) and transferred into the water flux in LMH (Eq. 3). RSF was calculated from the mass balance of NaCl (Eq. 4).
where \({J}_{w}\) is the water flux in LMH, \({\mathrm{J}}_{\mathrm{s},\mathrm{ TFC}-\mathrm{HF}}\) is the RSF in mmol h−1 m−2, and \({W}_{\mathrm{initial feed}}\) and \({W}_{\mathrm{final feed}}\) are the buffer tank weights at the beginning of the experiment and when the required recovery rate was reached, respectively. \({C}_{\mathrm{initial feed}}\) and \({C}_{\mathrm{final feed}}\) are the feed tank’s initial and final concentrations. The final concentration was measured when the target volume reduction was obtained. A is the effective membrane area in m2, t is the experiment time in hours, and \({Mw}_{s}\) is the solute molecular weight in mg/mmol−1.
The experiments on the synthetic feed solutions were duplicated to assess the data’s reproducibility. The results revealed that the experiment is reproducible, with absolute error values ranging from 0.24 to 3%.
2.5 Integrity test and cleaning
The TFC membrane’s fouling propensity was assessed by conducting two baseline trials using synthetic solutions before and after each experiment with the real PW feed. Pure tap water was used to flush the surface of the membrane for at least half an hour before each baseline test. Two chemical cleaning protocols were performed to remove the organic and inorganic contaminants from the membrane surface. Sodium hydroxide solution (250 mg L−1 NaOH) was utilized as a reagent to remove the organic foulants. The NaOH solution’s flow rate was maintained constant at 0.5 L min−1 for approximately 15 min at a pH of 11. The membrane surface was then flushed with pure water for 30 min at a flow rate of 0.5. A citric acid solution (2%) was circulated on the membrane surface for 15 min at a flow rate of 0.5 L min−1 and pH of 3, then the membrane surface was flushed again with tap water. Citric acid was used primarily to remove the inorganic contaminants such as calcium carbonate and calcium and iron phosphates.
2.6 Analytical methods
Water samples from the feed inlet, feed outlet (concentrate), DS inlet, and DS outlet (diluted) were collected for water quality measurements during pilot testing under the real PW stream. A total of 18 water samples were analyzed for turbidity (Hach Turbidmeter, USA), pH (Titrando, Switzerland), and chlorine amount (Hach, USA). The total organic and inorganic contents were also analyzed (TOC-L, Shimadzu), and ion chromatography (Thermo Scientific, USA) was used to measure the cation and anion concentrations of the four water streams.
3 Results and discussion
3.1 OC unit operation with synthetic waters
3.1.1 Water flux and volume reduction rate
Membrane performance when subjected to the synthetic solutions is illustrated in Fig. 4, where the achievable water flux values can be related to the feed recovery rates. Figure 4 indicates a descending water flux trend when raising the target volume reduction rate from 60 to 90%. The Aromatec membrane achieved a flux of 11.45 LMH with a 60% feed recovery, which then declined to 9.31, 8.77, and 6.44 LMH with recovery rates of 68%, 75%, and 90%, respectively. Higher recoveries were obtained by running the system at higher feed concentration levels. The diminished osmotic driving force explains the lower water fluxes at higher feed recoveries [42]. As freshwater permeates through the membrane barrier, the FS is concentrated while the DS is diluted, resulting in a higher buffer feed tank solution concentration and a reduced osmotic pressure difference across the membrane.
Identical flux trends for all feed recovery rates are illustrated in Fig. 4b; however, the time required to reach higher recoveries was longer than lower recoveries, due primarily to the Aromatec membrane’s operating procedure in recirculation mode, which results in an increasing buffer feed tank solution concentration. Lower fluxes were observed at higher feed recoveries because of the reduced osmotic driving force at higher buffer feed tank solution concentrations. The obtained results confirmed the programmed data acquisition system’s capability to accurately measure water flux and other operational parameters.
The ability to obtain various feed recoveries during pilot testing was linked directly to the buffer tank solution concentration change with time at constant FS and DS stream flow rates (Fig. 5). The initial conductivity of the 2000 mg L−1 feed solution was approximated by 4400 µS cm−1, which increased to 19,460 µS cm−1 when the recovery reached a maximum of 90%. The 60% feed recovery was first achieved after 2.2 h, where the feed tank conductivity was 8377 µS cm−1. The recovery then gradually increased to 68% and 75% when the feed tank conductivity reached 9805 and 11,450 µS cm−1, respectively.
3.1.2 Reverse solute flux
The RSF values recorded during Aromatec pilot testing at different volume reductions are illustrated in Fig. 6. These values were estimated from the FS and DS stream conductivity measurements. RSF is a quantitative measure of the solute transferred from the DS to the feed solution side. Lower RSF values are favorable to minimize the need for periodic DS replenishment [43]. A maximum RSF of 38 mmol h−1 m−2 was recorded at the lowest volume reduction value of 60% during pilot testing. The operation at the greatest volume reduction, 90%, generated the minimum RSF, 76% lower than the RSF obtained at a 60% feed recovery. Operating the pilot unit at a modest volume reduction of 75% resulted in an RSF of 11.09 mmol h−1 m−2. This value is 71% lower than that observed at 60% recovery, and 22% higher than the value recorded at 90%. These findings indicate a direct relationship between water flux and RSF. The operation at the lowest volume reduction of 60%, when the water flux was the highest, resulted in the greatest reverse solute transport RSF. Similar observations were reported by Heo et al. [44], who recorded a consistent increase in RSF with water flux.
The specific reverse solute flux (SRSF) was estimated and plotted in Fig. 6 to analyze the membrane’s performance better. SRSF represents the amount of the DS lost from the feed normalized to the amount of water that can pass through the membrane (Js/Jw). The SRSF decreased when increasing the feed recovery up to 75%, then a slight increase in SRSF was observed at a 90% recovery rate. This phenomenon can be explained by the fact that the SRSF depends on the rate of change of both Js and Jw when the volume reduction rate is altered [45, 46]. A 26% Jw was recorded when the volume reduction rate was raised from 75 to 90%, while the Js decreased by only 18%, resulting in a higher SRSF with a feed recovery of 90%. Escalating the volume reduction rate from 68 to 75% led to a lower Jw reduction, 5.7%, compared to the RSF reduction of 21%. A 16% lower SRSF was recorded at a 75% volume reduction as a result, compared to that observed at 68%; therefore, the operation at a 75% feed recovery resulted in the lowest SRSF of 1.26 mmol L−1. A lower SRSF value is favorable for a lower DS loss when it is normalized to the amount of water passing through the membrane.
The operating conditions appear to have a vital role in the pilot unit’s performance. A trade-off exists between achieving high membrane flux and volume reduction while minimizing the amount of DS loss (RSF). The highest flux and RSF values were recorded at the lowest volume reduction rate of 60%. The TFC membrane can achieve the lowest RSF when the recovery rate is the highest, 90%, and the water flux is at its minimum value. Higher imposed water fluxes can result in a greater membrane fouling risk; therefore, it is likely that the operation at an intermediate feed recovery rate, such as 75%, can provide the most favorable membrane performance since a good membrane flux (8.76 LMH) and the minimum SRSF (1.26 mmol L−1) were obtained at a lower risk of exposure to fouling.
3.2 Membrane performance under real PW
3.2.1 Water recovery, flux, and RSF
Figure 7 illustrates the membrane performance under the real PW stream at a 75% volume reduction for 48 h of operation. An initial membrane flux of 12.8 LMH was observed at the beginning of the trial; however, when the feed recovery reached 75% after approximately 2.8 h, a stable LMH flux of 10 was obtained. The membrane flux declined slightly to 9.6 LMH during the remaining 45 h of operation. The initial flux decline, from 12.8 to 10.0 LMH, before reaching 75% recovery can be ascribed to the lower reduced driving force, or osmotic gradient, between FS and DS due to the continuous feed concentration in the buffer tank. The lower flux recorded after maintaining 75% feed recovery likely results from contaminant accumulation when the real wastewater is introduced to the membrane.
The attainable water flux for the OC process is primarily determined by the osmotic pressure driving force across the membrane, controlled by FS and DS concentrations. Higher water flux is obtained when the concentration difference between these two streams increases. Minier-Matar and coworkers [27] demonstrated that the TFC membrane could achieve a water flux of 17.5 LMH at a 50% volume reduction rate at bench-scale, approximately 45% higher than the flux recorded in our study. The higher flux values are primarily attributed to the higher NaCl DS, or brine, concentrations used in their study. Draw solutions with higher NaCl concentrations possess greater osmotic pressures; therefore, they can provide a higher driving force for water permeation. The DS type and concentration are critical for obtaining a reasonable high membrane flux for the OC unit. The feed solution quality also controls the achievable water flux. A pre-treated effluent with a low conductivity value (around 2000 mg L−2) was utilized as FS in our trial program; however, the attainable membrane fluxes and dilution rates might be significantly lower than the values recorded in our study if the OC unit is subjected to a high salinity feed. OC is generally favorable for low salinity waste stream volume reduction or feed concentration applications.
The RSF exhibited by the TFC Aromatec membrane was estimated based on the initial feed water’s conductivity (2155 µS cm−1) and the feed’s conductivity (~ 7690 µS cm−1) when a 75% recovery rate was achieved. The Aromatec module had an RSF of 19.6 mmol m−2 h−1, equivalent to 1145.4 mg m−2 h−1. The RSF and SRSF obtained in our pilot testing were compared with values reported for other TFC and CTA membranes (Table 4). The RSF varied with membrane materials, feed and DS salinity, and membrane hydrodynamics or solution flow velocities. CTA membranes had a higher RSF than TFC, while reduced RSF values were obtained at higher FS and DS flow rates due to the module’s enhanced solution mixing conditions. The RSF and SRSF values recorded in this study are comparable to similar findings from the literature [21, 27]. The analytical water quality results were also used to perform mass balance calculations to determine the forward organic flux for the Aromatec module. There was approximately 1.1 mg m−2 h−1 organic flux from the FS to the DS side, based on the obtained calculations. The organic flux can be estimated at 0.1 if this value is divided by the total water permeate flux. Similar findings were reported for other CTA FO membranes [47, 48].
3.2.2 Membrane fouling
Two baseline tests, before and after the real wastewater experiments, were performed using the synthetically prepared feed and draw solutions. The results of the initial and final baselines were compared to assess the Aromatec module’s fouling propensity. Figure 8 indicates that the water flux had a value of 8.2 LMH before the membrane was subjected to the PW feed; however, a higher water flux (10 LMH) was generated by the OC unit with the real PW. This greater flux value can be explained by the increased concentration difference, or driving force, due to the PW stream’s lower feed salinity compared to the synthetic solution (2155 c.f 4000 µS/cm). Figure 8 also reveals that the final baseline test had a flux of 7.35 LMH, approximately 10.36% lower than the flux recorded for the initial baseline (8.20 LMH). This lower flux was likely caused by membrane fouling; which was influenced by many factors, including water chemistry and operating conditions (flow rate 1.5 L/min). The increased flow impacted the rate of organic carbons and minerals scaling. However, it was unclear which type of fouling, organic or inorganic, caused the flux decline. We applied OLI simulations to evaluate the scaling tendency of the inorganic contaminants that exist in the PW feed. Results demonstrated that some compounds, such as calcium phosphate, can precipitate on the surface of the membrane under the tested conditions; however, the probability of Ca3(PO4)2 precipitation is low if the pH of the outlet FS is lower than 6.5. The concentrate stream’s pH was consistently above 7; therefore, the flux deterioration observed in Fig. 8 was ascribed to inorganic scaling on the membrane’s surface.
A flux decline of 15% was reported due to organic fouling during the previous OC bench-scale study [14]. The feed stream used in that study was a mixture of produced and process water with a TOC of 120 mg/L; however, the FS had a lower TOC content (~ 10 mg/L) than in the current study. Our hypothesis that organic fouling has a negligible impact on the water flux is valid, and it is unlikely that some organics would attach to the surface of the membrane with this low concentration.
3.2.3 Membrane integrity and cleaning efficacy
The membrane was cleaned with two different reagents to further confirm the existence of the insignificant organic fouling, each of which targeted the removal of either the organic or inorganic contaminants [51]. A baseline trial was conducted after each cleaning cycle. NaOH (250 mg L−1) was used as a cleaning reagent to remove possible organic foulants, while a 2% citric acid solution was used to remove the inorganic compounds during the second cleaning cycle. The most relevant observation (Fig. 9) is that the TFC membrane exhibited comparable water fluxes (6.9–7.3 LMH) for the baseline experiments before and after cleaning with NaOH. The insignificant difference (< 5%) in the water fluxes of these two baselines can be attributed to the slightly different operating temperatures of the two tests. A significantly higher water flux (8.25 LMH) was observed after cleaning the membrane with the citric acid solution. Citric acid is an effective cleaning reagent that can remove phosphate salts and calcium carbonate from the surface of the membrane, as reported in other previous studies [52, 53]; therefore, the flux deterioration observed in Fig. 7 was attributed primarily to inorganic scaling. These findings are in good agreement with OLI simulation results, which indicated the likelihood of ion deposition on the membrane’s surface.
The OC membranes had a low fouling propensity; however, their flux decline was not completely recoverable by simple water flushing only, and chemical cleaning was necessary to recover the initial flux. Citric acid exhibited efficacy in cleaning the inorganic foulants and restoring the initial flux; however, other reagents, such as SDS and ethylenediamine tetraacetic acid (EDTA), will be needed when the feed stream has high TOC/organic content.
3.2.4 Water quality
Table 5 lists the water characteristics of the four membrane streams: feed inlet, feed outlet/concentrate, draw solution inlet, and DS outlet/diluted. These water samples were analyzed to determine the contaminant concentrations. Table 5 also presents the averages of five samples, obtained during the 48 h of the unit operation. The water samples had a pH of less than 8, and a Cl2 content less than 0.1 mg L−1, which complies with the pH and chlorine tolerance values specified by the membrane module supplier. The turbidity was less than 0.5 NTU in all water samples because both feed and draw solutions were filtered with a 5-µm three-stage filter. The pilot unit produced a concentrate stream with less than 31.65 mg L−1 TOC. A small amount of the TOC was lost from the FS to the DS stream due to the organics flux; nonetheless, the DS inlet and outlet had a TOC concentration less than 1 mg L−1. There was a similar small loss of sodium and chloride from the DS, which can be explained by the RSF. The TOC and inorganic contaminant concentrations were very low in the diluted DS stream, which is intended to be discharged directly into the ocean. Direct discharge into the ocean appears to be a feasible option since the water quality of the diluted DS stream is just diluted seawater or brine without significant amounts of organics or inorganics.
4 Conclusions
The current study presents an application-oriented investigation of the FO technology for injected wastewater volume reductions. The robustness of the osmotic concentration process was examined at pilot scale using synthetic and real PW feed solutions. The pilot testing results revealed the following:
-
i.
The Aromatec module achieved various feed recoveries up to 90%; however, the attainable water flux was lower at higher feed recoveries.
-
ii.
Operation at an intermediate feed recovery rate, such as 75%, was characterized as the best operating condition for the pilot unit since it had the lowest SRSF (1.26 mmol L−1) at a reasonably high flux of 8.76 LMH; therefore, the OC performance with the real wastewater stream was examined at a 75% feed recovery.
-
iii.
Trials on the real wastewater confirmed the stability of the OC technology for a relatively long period (48 h). The TFC HF membrane achieved a 75% feed volume reduction with a stable flux.
-
iv.
The TFC Aromatec module demonstrated a stable water flux of 10 LMH and RSF of 19.6 mmol m−2 h −1. Membrane integrity tests revealed a slight decline of approximately 10.36% in the membrane water flux due to inorganic fouling, which was demonstrated by simulation outcomes and membrane cleaning.
-
v.
The chemical cleaning effectiveness was examined for mitigating organic and inorganic fouling. Results indicated that inorganic scaling was the predominant type of fouling, and cleaning with a citric acid solution could restore the initial flux.
-
vi.
Analytical water sample measurements revealed insignificant contaminant concentrations in the diluted DS, which allows for the direct discharge of this stream into the ocean.
-
vii.
The current study demonstrated that the OC technology could provide a feasible solution for wastewater volume minimization before end disposal. Beneficial environmental impacts arise from brine dilution before discharging into the sea; however, the OC approach is hindered by limited experience with full-scale installations.
Data Availability
The raw data required to reproduce the above findings cannot be shared as the data also forms part of an ongoing study.
References
J. Cleland, World population growth; Past, present and future. Environ. Resour. Econ. 55, 543–554 (2013). https://doi.org/10.1007/s10640-013-9675-6
S. Adham, A. Hussain, J. Minier-Matar, A. Janson, R. Sharma, Membrane applications and opportunities for water management in the oil & gas industry. Desalination 440, 2–17 (2018). https://doi.org/10.1016/j.desal.2018.01.030
G. Han, J.S. de Wit, T.S. Chung, Water reclamation from emulsified oily wastewater via effective forward osmosis hollow fiber membranes under the PRO mode. Water Res. 81, 54–63 (2015). https://doi.org/10.1016/j.watres.2015.05.048
H. Simpson, S. Lester, Deep well injection: An explosive issue, (2009). Center for Health, Environment, and Justice. https://www.chej.org
A.M. Awad, R. Jalab, M. S.Nasser, I. A.Hussein, Membrane-based treatment of petroleum wastewater, in: ed. M.H. El-Naas, A. Banerjee (Pet. Ind. Wastewater, Elsevier, 2022), pp. 103–122. https://doi.org/10.1016/B978-0-323-85884-7.00003-5.
M. Shahbaz, N. Rashid, J. Saleem, H. Mackey, G. McKay, T. Al-Ansari, A review of waste management approaches to maximise sustainable value of waste from the oil and gas industry and potential for the State of Qatar. Fuel. 332, 126220 (2023). https://doi.org/10.1016/j.fuel.2022.126220
Q. She, R. Wang, A.G. Fane, C.Y. Tang, Membrane fouling in osmotically driven membrane processes: A review. J. Memb. Sci. 499, 201–233 (2016). https://doi.org/10.1016/j.memsci.2015.10.040
R. Jalab, A.M. Awad, M.S. Nasser, J. Minier-matar, S. Adham, Pilot-scale investigation of flowrate and temperature influence on the performance of hollow fiber forward osmosis membrane in osmotic concentration process. J. Environ. Chem. Eng. 8, 104494 (2020). https://doi.org/10.1016/j.jece.2020.104494
G. Han, C.Z. Liang, T.S. Chung, M. Weber, C. Staudt, C. Maletzko, Combination of forward osmosis (FO) process with coagulation/flocculation (CF) for potential treatment of textile wastewater. Water Res. 91, 361–370 (2016). https://doi.org/10.1016/j.watres.2016.01.031
G. Han, S.S. Chan, T.S. Chung, Forward osmosis (FO) for water reclamation from emulsified oil/water solutions: Effects of membrane and emulsion characteristics. ACS Sustain. Chem. Eng. 4, 5021–5032 (2016). https://doi.org/10.1021/acssuschemeng.6b01402
W.L. Ang, A. Wahab Mohammad, D. Johnson, N. Hilal, Forward osmosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J. Water Process Eng. 31, 100886 (2019). https://doi.org/10.1016/j.jwpe.2019.100886
A. Subramani, J.G. Jacangelo, Emerging desalination technologies for water treatment: A critical review. Water Res. 75, 164–187 (2015). https://doi.org/10.1016/j.watres.2015.02.032
I. Ibrar, S. Yadav, O. Naji, A.A. Alanezi, N. Ghaffour, S. Déon, S. Subbiah, A. Altaee, Development in forward Osmosis-Membrane distillation hybrid system for wastewater treatment, Sep. Purif. Technol. 286 (2022). https://doi.org/10.1016/j.seppur.2022.120498
D.L. Shaffer, J.R. Werber, H. Jaramillo, S. Lin, M. Elimelech, Forward osmosis: Where are we now? Desalination 356, 271–284 (2015). https://doi.org/10.1016/j.desal.2014.10.031
R. Valladares Linares, Z. Li, S. Sarp, S.S. Bucs, G. Amy, J.S. Vrouwenvelder, Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 66, 122–139 (2014). https://doi.org/10.1016/j.watres.2014.08.021
B.D. Coday, P. Xu, E.G. Beaudry, J. Herron, K. Lampi, N.T. Hancock, T.Y. Cath, The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 333, 23–35 (2014). https://doi.org/10.1016/j.desal.2013.11.014
B.D. Coday, T.Y. Cath, Forward osmosis: Novel desalination of produced water and fracturing flowback. J. Am. Water Works Assoc. 106, 37–38 (2014). https://doi.org/10.5942/jawwa.2014.106.0016
X.M. Li, B. Zhao, Z. Wang, M. Xie, J. Song, L.D. Nghiem, T. He, C. Yang, C. Li, G. Chen, Water reclamation from shale gas drilling flow-back fluid using a novel forward osmosis-vacuum membrane distillation hybrid system. Water Sci. Technol. 69, 1036–1044 (2014). https://doi.org/10.2166/wst.2014.003
R.A. Maltos, J. Regnery, N. Almaraz, S. Fox, M. Schutter, T.J. Cath, M. Veres, B.D. Coday, T.Y. Cath, Produced water impact on membrane integrity during extended pilot testing of forward osmosis – reverse osmosis treatment. Desalination 440, 99–110 (2018). https://doi.org/10.1016/j.desal.2018.02.029
A.M. Awad, R. Jalab, J. Minier-Matar, S. Adham, M.S. Nasser, S.J. Judd, The status of forward osmosis technology implementation. Desalination (2019). https://doi.org/10.1016/j.desal.2019.03.013
J. Minier-Matar, A. Hussain, A. Janson, R. Wang, A.G. Fane, S. Adham, Application of forward osmosis for reducing volume of produced/Process water from oil and gas operations. Desalination 376, 1–8 (2015). https://doi.org/10.1016/j.desal.2015.08.008
M. Salamanca, L. Palacio, A. Hernandez, M. Peña, P. Pr, Evaluation of forward osmosis and low-pressure reverse osmosis with a tubular membrane for the concentration of municipal wastewater and the production of biogas. Membranes. 13, 266 (2023). https://doi.org/10.3390/membranes13030266
L. Chekli, J. Eun, I. El, Y. Kim, S. Phuntsho, S. Li, N. Ghaffour, T. Leiknes, H. Kyong, Fertilizer drawn forward osmosis process for sustainable water reuse to grow hydroponic lettuce using commercial nutrient solution. Sep. Purif. Technol. 181, 18–28 (2017). https://doi.org/10.1016/j.seppur.2017.03.008
D. Wang, J. Zhang, J. Li, W. Wang, H.K. Shon, H. Huang, Y. Zhao, Z. Wang, Inorganic scaling in the treatment of shale gas wastewater by fertilizer drawn forward osmosis process. Desalination. 521, 115396 (2022). https://doi.org/10.1016/j.desal.2021.115396
G. Jeong, D. Jang, H. Lee, A. Jang, Effects of feed solution chemistry on membrane scaling in fertilizer-drawn forward osmosis process for sustainable agricultural reuse. Desalination. 545, 116150 (2023). https://doi.org/10.1016/j.desal.2022.116150
T.Y. Cath, N.T. Hancock, C.D. Lundin, C. Hoppe-jones, J.E. Drewes, A multi-barrier osmotic dilution process for simultaneous desalination and purification of impaired water. J. Memb. Sci. 362, 417–426 (2010). https://doi.org/10.1016/j.memsci.2010.06.056
J. Minier-Matar, A. Santos, A. Hussain, A. Janson, R. Wang, A.G. Fane, S. Adham, Application of hollow fiber forward osmosis membranes for produced and process water volume reduction: An osmotic concentration process. Environ. Sci. Technol. 50, 6044–6052 (2016). https://doi.org/10.1021/acs.est.5b04801
W. Suwaileh, N. Pathak, H. Shon, N. Hilal, Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination. 485, 114455 (2020). https://doi.org/10.1016/j.desal.2020.114455
B. Corzo, T. de la Torre, C. Sans, E. Ferrero, J.J. Malfeito, Evaluation of draw solutions and commercially available forward osmosis membrane modules for wastewater reclamation at pilot scale. Chem. Eng. J. 326, 1–8 (2017). https://doi.org/10.1016/j.cej.2017.05.108
N. Akther, A. Sodiq, A. Giwa, S. Daer, H.A. Arafat, S.W. Hasan, Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 281, 502–522 (2015). https://doi.org/10.1016/j.cej.2015.05.080
Y. Chun, F. Zaviska, S.J. Kim, D. Mulcahy, E. Yang, I.S. Kim, L. Zou, Erratum: Corrigendum to: “Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water” (Desalination (2016) 394 (91–100)). Desalination. 402, 185–187 (2017). https://doi.org/10.1016/j.desal.2016.10.010.
N.T. Hancock, P. Xu, M.J. Roby, J.D. Gomez, T.Y. Cath, Towards direct potable reuse with forward osmosis : Technical assessment of long-term process performance at the pilot scale. J. Memb. Sci. 445, 34–46 (2013). https://doi.org/10.1016/j.memsci.2013.04.056
N.R. Hutchings, E.W. Appleton, R.A. McGinnis, Making high quality frac water out of oilfield waste, 19–22 (2011). https://doi.org/10.2118/135469-ms
K.L. Hickenbottom, N.T. Hancock, N.R. Hutchings, E.W. Appleton, E.G. Beaudry, P. Xu, T.Y. Cath, Forward osmosis treatment of drilling mud and fracturing wastewater from oil and gas operations. Desalination 312, 60–66 (2013). https://doi.org/10.1016/j.desal.2012.05.037
L. Zhu, C. Ding, T. Zhu, Y. Wang, A review on the forward osmosis applications and fouling control strategies for wastewater treatment. Front. Chem. Sci. Eng. 16, 661–680 (2022). https://doi.org/10.1007/s11705-021-2084-4
T. Alamoudi, M.S. Nawaz, M. Obaid, Y. Jin, S. Soukane, H.S. Son, V. Gudideni, A. Al-Qahtani, N. Ghaffour, Optimization of osmotic backwashing cleaning protocol for produced water fouled forward osmosis membranes. J. Memb. Sci. 663, 121013 (2022). https://doi.org/10.1016/j.memsci.2022.121013
M. Yan, M. Shao, J. Li, N. Jiang, Y. Hu, W. Zeng, M. Huang, Antifouling forward osmosis membranes by ε-polylysine mediated molecular grafting for printing and dyeing wastewater: Preparation, characterization, and performance. J. Memb. Sci. 668, 121288 (2023). https://doi.org/10.1016/j.memsci.2022.121288
R. Lakra, M.R. Bilad, M. Balakrishnan, S. Basu, Development of high-performance CuBTC MOF-based forward osmosis (FO) membranes and their cleaning strategies. Chem. Eng. Res. Des. 190, 566–579 (2023). https://doi.org/10.1016/j.cherd.2023.01.005
M. Golgoli, M. Khiadani, T.K. Sen, A. Razmjou, M.L. Johns, M. Zargar, Synergistic effects of microplastics and organic foulants on the performance of forward osmosis membranes. Chemosphere. 311, 136906 (2023). https://doi.org/10.1016/j.chemosphere.2022.136906
A.M. Awad, R. Jalab, M.S. Nasser, M. El-Naas, I.A. Hussein, J. Minier-Matar, S. Adham, Evaluation of cellulose triacetate hollow fiber membrane for volume reduction of real industrial effluents through an osmotic concentration process: A pilot-scale study. Environ. Technol. Innov. 24, 101873 (2021). https://doi.org/10.1016/j.eti.2021.101873
G. Han, J. Zhou, C. Wan, T. Yang, T.S. Chung, Investigations of inorganic and organic fouling behaviors, antifouling and cleaning strategies for pressure retarded osmosis (PRO) membrane using seawater desalination brine and wastewater. Water Res. 103, 264–275 (2016). https://doi.org/10.1016/j.watres.2016.07.040
R. Jalab, A.M. Awad, M.S. Nasser, I.A. Hussein, F. Almomani, J. Minier-Matar, S. Adham, Investigation of thin-film composite hollow fiber forward osmosis membrane for osmotic concentration: A pilot-scale study. Korean J. Chem. Eng. 39, 178–188 (2022). https://doi.org/10.1007/s11814-021-0935-9
X. Wu, X. Zhang, H. Wang, Z. Xie, Smart utilisation of reverse solute diffusion in forward osmosis for water treatment: A mini review. Sci. Total Environ. 873, 162430 (2023). https://doi.org/10.1016/j.scitotenv.2023.162430
J. Heo, K.H. Chu, N. Her, J. Im, Y.G. Park, J. Cho, S. Sarp, A. Jang, M. Jang, Y. Yoon, Organic fouling and reverse solute selectivity in forward osmosis: Role of working temperature and inorganic draw solutions. Desalination 389, 162–170 (2016). https://doi.org/10.1016/j.desal.2015.06.012
G. Han, S. Zhang, X. Li, N. Widjojo, T.S. Chung, Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 80, 219–231 (2012). https://doi.org/10.1016/j.ces.2012.05.033
D. Ma, S.B. Peh, G. Han, S.B. Chen, Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal-organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection. ACS Appl. Mater. Interfaces. 9, 7523–7534 (2017). https://doi.org/10.1021/acsami.6b14223
M. Sauchelli, G. Pellegrino, A. D’Haese, I. Rodríguez-Roda, W. Gernjak, Transport of trace organic compounds through novel forward osmosis membranes: Role of membrane properties and the draw solution. Water Res. 141, 65–73 (2018). https://doi.org/10.1016/j.watres.2018.05.003
S. Zou, M. Qin, Z. He, Tackle reverse solute flux in forward osmosis towards sustainable water recovery: Reduction and perspectives. Water Res. 149, 362–374 (2019). https://doi.org/10.1016/j.watres.2018.11.015
E.A. Bell, T.E. Poynor, K.B. Newhart, J. Regnery, B.D. Coday, T.Y. Cath, Produced water treatment using forward osmosis membranes: Evaluation of extended-time performance and fouling. J. Memb. Sci. 525, 77–88 (2017). https://doi.org/10.1016/j.memsci.2016.10.032
S. Zhao, J. Minier-Matar, S. Chou, R. Wang, A.G. Fane, S. Adham, Gas field produced/process water treatment using forward osmosis hollow fiber membrane: Membrane fouling and chemical cleaning. Desalination 402, 143–151 (2017). https://doi.org/10.1016/j.desal.2016.10.006
M. Bagherzadeh, M. Nikkhoo, M.M. Ahadian, M. Amini, Novel thin-film nanocomposite forward osmosis membranes modified with WS2/CuAl LDH nanocomposite to enhance desalination and anti-fouling performance. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02547-6
B. Agnihotri, A. Sharma, A.B. Gupta, Characterization and analysis of inorganic foulants in RO membranes for groundwater treatment. Desalination. 491, 114567 (2020). https://doi.org/10.1016/j.desal.2020.114567
R. Bogati, C. Goodwin, K. Marshall, K.T. Leung, B.Q. Liao, Optimization of chemical cleaning for improvement of membrane performance and fouling control in drinking water treatment. Sep. Sci. Technol. 50, 1835–1845 (2015). https://doi.org/10.1080/01496395.2015.1009116
Acknowledgements
The findings achieved herein are solely the responsibility of the authors. The authors would like to thank Dan Jerry Cortes from Qatar University and Arnold Janson from ConocoPhillips, Qatar, for providing useful information for this paper.
Funding
Open Access funding provided by the Qatar National Library. The authors would also like to acknowledge the support of the Qatar National Research Fund (a member of Qatar Foundation) through Grants # NPRP10-0118170191 and NPRP12S-0306–190247.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
There are no competing financial interests or personal relationships that could influence this research work. Also, the manuscript is not submitted simultaneously to another journal.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Awad, A.M., Jalab, R., Nasser, M.S. et al. Pilot scale evaluation of thin film composite membranes for reducing wastewater volumes: osmotic concentration process. emergent mater. 7, 619–632 (2024). https://doi.org/10.1007/s42247-023-00495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00495-y