Abstract
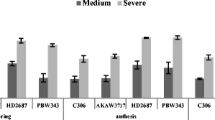
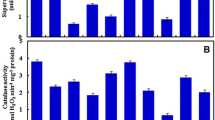
Variability in enzymatic and non enzymatic antioxidants could be useful for breeding genotypes tolerant to different abiotic stresses. The objective of present study was to determine the variability in enzymatic and non enzymatic antioxidants in wheat at three different stages of development including leaves of vegetative stage, flag leaf stage after 5 days of anthesis and in mature grains. Forty wheat genotypes including 10 commercial cultivars, 5 rainfed cultivars, 17 advanced breeding lines and 8 Australian cultivars were raised under irrigated conditions. At vegetative stage, high activity of superoxide dismutase (SOD), peroxidase (POX), glutathione reductase (GR) and ascorbate peroxidase (APX) and low hydrogen peroxide (H2O2) content was observed in many of the advanced breeding lines, while high proline and low malondialdehyde (MDA) content was observed in many commercial cultivars. In flag leaf after 5 days of anthesis higher activity of SOD and APX was observed in many of rain-fed cultivars; many commercial cultivars showed high activity of POX and GR while low H2O2 content was observed in many of Australian cultivars. Ruby, Binnu and Datatine have low H2O2 and MDA content so they could be used for studying tolerance towards different types of abiotic stresses. PBW 550 showed high antioxidant activity in leaves during vegetative and flag leaf stage, it could be worthwhile to study the performance of this cultivar under different abiotic stresses. Variability was also observed in mature grains of different wheat genotypes. In mature grains high proline content was observed in many of rain-fed cultivars while less GR, CAT and APX activity was observed in many of Australian genotypes. Mature grains of wheat genotypes PBW 644, PBW542, DBW 16, DBW 17, WH 1021, PBW 676, BWL 73 and PBW 175 have high activity of APX, GR and some have high proline content. In general genotypes with high enzymatic antioxidants and low H2O2 and MDA content may be useful for studying tolerance towards different abiotic stresses. Genotypes with high antioxidants were identified for possible use in wheat breeding programme.
Similar content being viewed by others
References
Almeselmani, M., Deshmukh, P. S., Sairam, R. K., Kushwaha, S. R., & Singh, T. P. (2006). Protective role of antioxidant enzymes under high temperature stress. Plant Science, 171, 382–388.
Asada, K. (1999). The water–water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Biology, 50, 601–639.
Ashraf, M. A., Asharf, M., & Ali, Q. (2010). Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Lipid peroxidation and phenolic contents. Pakistan Journal of Botany, 42, 559–565.
Bates, L. S. (1973). Rapid determination of free proline content for water-stress studies. Plant and Soil, 39, 205–207.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200.
Chance, B., & Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods in Enzymology, 2, 764–775.
Chaves, M. M., Maroco, J. P., & Pereira, J. (2003). Understanding plant responses to drought from genes to the whole plant. Functional Plant Biology, 30, 239–264.
Csiszar, J., Bernadett, P., Zsuzsanna, K., Laszlo, E., & Irma, T. (2008). Peroxidase activities in root segments of wheat genotypes under osmotic stress. Acta Biologica Szegediensis, 52, 155–156.
Devi, R. (2008). A study on the antioxidative system in wheat under water deficient conditions. Ph.D. Dissertation. Punjab Agricultural University, Ludhiana, India.
Doke, N., & Scandalios, J. G. (1997). The oxidative burst: roles in signal transduction and plant stress. In: Oxidative stress and the molecular biology of antioxidant defenses (785–13). New Spring Harbor Laboratory Press.
Esfandiari, E., Shekari, F., Shekari, F., & Esfandiari, M. (2007). The effect of salt stress on antioxidant enzyme activity and lipid peroxidation on the wheat seedling. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 35, 48–56.
Evenson, R. E., & Gollin, D. (2003). Accessing the impact of green revolution, 1960–2000. Science, 300, 758–762.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
Hasheminasab, H., Assad, M. T., Aliakbari, A., & Sahhafi, R. (2012). Influence of drought stress on oxidative damage and antioxidant defense system in tolerant and susceptible wheat genotypes. Journal of Agricultural Science, 4, 20–30.
Huseynova, I. M., Suleymanov, S. Y., & Rustamova, M. S. (2010). Response of photosynthetic apparatus and antioxidant defense systems in Triticum aestivum L. genotype subjected to drought stress. Biological Sciences, 65, 49–59.
Kahrizi, S., Sedighi, M., & Sofalian, O. (2012). Effect of salt stress on proline and activity of antioxidant enzymes in ten duram wheat cultivars. Annals of Biological Research, 3, 3870–3874.
Kaur, S., Gupta, A. K., Kaur, N., Sandhu, J. S., & Gupta, S. K. (2009). Antioxidative enzymes and sucrose synthase contribute to cold stress tolerance in chickpea. Journal of Agronomy and Crop Science, 195, 393–397.
Khan, M. A., Shirazi, M. U., Khan, M. A., Mujtaba, S. M., Islam, E., Mumtaz, S., Shereen, A., Ansari, R. V., & Ashraf, M. Y. (2009). Role of proline, K/Na ratio and chlorophyll content in salt tolerance of wheat (Triticum aestivum L.). Pakistan Journal of Botany, 41, 633–638.
Khanna-Chopra, R., & Selote, D. S. (2007). Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than susceptible wheat cultivar under field conditions. Environmental and Experimental Botany, 60, 276–283.
Kotchoni, O. S. (2004). Molecular and physiological analysis of transgenic Arabidopsis plants expressing dehydrogenase genes. Ph.D. Thesis, Bonn, Germany.
Kumar, S. (2007). Identification of biochemical markers of drought tolerance in wheat. M.Sc. Thesis. Punjab Agricultural University, Ludhiana, India.
Law, M. Y., Charles, S. A., & Halliwell, B. (1983). Glutathione and ascorbic acid in spinach chloroplasts. Biochemical Journal, 210, 899–903.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Marklund, S., & Marklund, G. (1974). Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 169–174.
Mandhania, S., Madan, S., & Sheokand, S. (2010). Differential response in salt tolerant and sensitive genotypes of wheat in terms of ascorbate, carotenoids, proline and plant water relations. Asian Journal of Experimental Biological Sciences, 1, 742–797.
Mantri, N., Patade, V., Penna, S., Ford, R., & Pang, E. (2012). Abiotic stress responses in plants: Present and future. In: P. Ahmad and M. N. V. Prasad (Eds.), Abiotic stress responses in plants: Metabolism, productivity and sustainability (pp. 1–19). doi: https://doi.org/10.1007/978-1-4614-0634-1_1
Nakano, Y., & Asada, K. (1987). Purification of ascorbate peroxidase in spinach choloroplasts: Its inactivation in ascorbate depleted medium and reactivation by monodehydroascorbate radical. Plant and Cell Physiology, 28, 131–140.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Quan, L. J., Zhang, B., Shi, W. W., & Li, H. Y. (2008). Hydrogen peroxide in plants a versatile molecule of the reactive oxygen species network. Journal of Integrative Plant Biology, 50, 2–18.
Reynolds, M. P., & Borlaugh, N. E. (2006). Impacts of breeding on international collaborative wheat improvement. Journal of Agricultural Science, 144, 3–17.
Sairam, R. K., Deshmukh, P. S., & Saxena, D. C. (1998). Role of antioxidant systems in wheat genotypes tolerance to water stress. Biologia Plantarum, 41, 387–394.
Shannon, L. M., Kay, E., & Lew, J. K. (1966). Peroxidase isoenzyme from horseradish roots.I.Isolation and physical properties. Journal of Biological Chemistry, 241, 2166–2172.
Shao, H. B., Liang, Z. S., & Shao, M. A. (2005). Dynamic changes of antioxidative enzymes of ten wheat genotypes at soil water deficits. Colloids and Surfaces B: Biointerfaces, 42, 187–195.
Sinha, A. K. (1971). Colorimetric assay of catalase. Analytical Biochemistry, 47, 389–394.
Valifard, M., Moradshahi, A., & Kholdebarin, B. (2012). Biochemical and physiological reponses of two wheat (Triticum aestivum) cultivars to drought stress applied at seedling stage. Journal of Agricultural Science and Technology, 14, 1567–1578.
Valko, M., Morris, H., & Cronnin, M. T. D. (2006). Metal, toxicity and oxidative stress. Current Medicinal Chemistry, 12, 1161–1162.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patil, P., Gupta, A.K., Bains, N.S. et al. Variability in enzymatic and non enzymatic antioxidants in wheat (Triticum aestivum L.) genotypes. Plant Physiol. Rep. 26, 428–442 (2021). https://doi.org/10.1007/s40502-021-00584-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-021-00584-2