Abstract
Introduction
This study compared outcomes of the iStent inject trabecular micro-bypass system versus the Hydrus Microstent in patients with primary open-angle glaucoma (POAG).
Methods
Forty subjects (80 eyes) with POAG were included in this single-center, retrospective, contralateral-eye analysis. All patients underwent phacoemulsification with either iStent inject or Hydrus implantation in one eye and the other device in the contralateral eye, with ≥ 3-month follow-up. In 58 eyes (27 iStent inject, 31 Hydrus) the surgery also included ab interno canaloplasty (ABiC). Twelve-month outcomes included intraocular pressure (IOP), medications, and adverse events. Subgroup analyses were completed for iStent inject versus Hydrus, and with versus without ABiC.
Results
At 12 months versus baseline, mean IOP reduced from 16.8 ± 3.7 to 13.6 ± 2.9 (p = 0.003) in iStent inject eyes, and from 18.1 ± 4.5 to 14.9 ± 3.2 mmHg (p = 0.003) in Hydrus eyes (between-group IOP reduction p = 0.582). Mean number of glaucoma medications reduced from 1.23 ± 0.97 to 0.30 ± 0.76 (p < 0.001) in iStent inject eyes and from 1.20 ± 1.02 to 0.39 ± 0.72 (p = 0.001) in Hydrus eyes (between-group medication reduction p = 0.943). At 12 months, 82.6% of iStent inject eyes and 73.9% of Hydrus eyes were medication-free versus 20.0% preoperatively in both groups (p < 0.0001 both groups). There were no statistically significant IOP or medication differences between iStent inject and Hydrus pre- or postoperatively, both in the overall cohort and in the with/without ABiC subgroups. Outcomes also were similar between eyes with/without ABiC in the overall cohort and in the iStent inject/Hydrus subgroups. There were no adverse events in the iStent inject group; two eyes in the Hydrus group had device-related complications requiring five additional surgeries (one Hydrus repositioning, one Hydrus exchange, one Hydrus removal, two goniotomies).
Conclusion
In this contralateral-eye comparison of iStent inject versus Hydrus, the groups had similar IOP and medication outcomes, regardless of stratification by ABiC completion. Eyes receiving Hydrus had more complications and subsequent surgeries.
Plain Language Summary
The present study contributes some of the first real-world data comparing iStent inject versus Hydrus Microstent implantation in combination with cataract surgery in opposite eyes (right or left) of the same patient (i.e., contralateral-eye study). The report also includes subgroup analyses of eyes with versus without ab interno canaloplasty (ABiC). There were no significant between-group differences in mean intraocular pressure or medication burden preoperatively or postoperatively for iStent inject versus Hydrus. The intraocular pressure and medication reductions versus the groups’ respective baselines were statistically similar as well. Finally, results remained similar for iStent inject versus Hydrus regardless of whether ABiC was completed, and were also similar when comparing eyes with ABiC versus without ABiC. In eyes receiving Hydrus, there was a greater incidence of complications and need for further surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Interventions to treat glaucoma, a major cause of blindness worldwide, must be evaluated for both their effectiveness and safety profiles. |
This dual-arm contralateral-eye retrospective dataset assessed 12-month outcomes following cataract surgery combined with either iStent inject (W) or Hydrus Microstent implantation in one eye and the other device in the opposite eye in patients with primary open-angle glaucoma. |
The iStent inject versus Hydrus results were stratified by whether concomitant ab interno canaloplasty (ABiC) was completed, and a comparison of eyes with versus without ABiC was performed as well. |
What was learned from the study? |
Significant intraocular pressure (IOP) and medication reductions versus baseline were achieved by both groups through 12 months. |
No significant between-group differences existed in the mean IOP or medication burden preoperatively or postoperatively. The IOP and medication reductions versus the groups’ respective baselines were statistically similar as well. |
iStent inject versus Hydrus outcomes remained similar after stratification for ABiC completion. Results were also similar when comparing eyes with or without ABiC. In eyes receiving Hydrus, there was a greater incidence of complications and need for further surgery. |
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide, affecting an estimated 76 million people [1, 2]. The primary treatment aim in glaucoma is the reduction of intraocular pressure (IOP). Even small decreases in IOP can significantly reduce the risk of glaucoma progression [3, 4], with the Early Manifest Glaucoma Trial showing an 11% decrease in disease progression risk per 1 mmHg of IOP reduction [5]. Historically, topical medications have been the first-line method to reduce IOP [6]. Topical treatments such as prostaglandin analogues and beta-blockers have the advantages of being relatively safe, cost-effective, and rapid-onset. However topical treatment has several limitations, including poor compliance, ocular surface discomfort [7], dry eye [8], pigmentary changes, hyperemia, orbital fat atrophy, and systemic side effects such as bradycardia and bronchospasm [9, 10].
An increasing number of surgical options are now available to treat glaucoma patients. Subconjunctival filtration surgeries such as trabeculectomy [11] and tube shunts [12] remain very effective in reducing IOP, particularly in advanced or secondary forms of glaucoma or in patients who cannot tolerate or are poorly compliant with topical glaucoma treatments. However, these procedures carry serious risks such as bleb-related infections and endophthalmitis, flat anterior chamber, corneal decompensation, suprachoroidal hemorrhage, bleb dysesthesia, hypotony maculopathy, conjunctival scarring, and bleb failure. For these reasons, they are often reserved for refractory and advanced glaucoma cases [13]. Patients who progress despite topical treatment can therefore be therapeutically challenging, as they require an escalation of treatment but may not warrant surgery with the aforementioned risks [14, 15].
Minimally invasive glaucoma surgery (MIGS) offers a potential solution to this treatment gap by providing a surgical option with a more favorable side effect profile than traditional filtration surgeries [16]. MIGS procedures can be classified based on their intended target tissue: trabecular, suprachoroidal, or subconjunctival [17]. Ab interno trabecular MIGS in particular, which acts upon the natural physiologic aqueous outflow pathway, can provide a surgical intervention with a favorable benefit-to-risk profile [16, 17] that takes into account patient comorbidities [18] and does not preclude future glaucoma surgery if needed [19].
In this retrospective contralateral study, we compared the clinical outcomes and safety profile of two trabecular bypass MIGS devices that improve aqueous outflow through the trabecular meshwork: the iStent inject trabecular micro-bypass system (either iStent inject or wide-flange iStent inject W; Glaukos Corporation, Aliso Viejo, CA, USA) and the Hydrus Microstent (Alcon, Fort Worth, TX, USA) when combined with phacoemulsification and in some cases ab interno canaloplasty (ABiC). Although there are some studies in the literature pertaining to iStent technologies and Hydrus in general, we are aware of only one study that compares two on-label uses of current-generation technology (i.e., iStent inject + phacoemulsification vs. Hydrus + phacoemulsification): the scientifically rigorous Fight Glaucoma Blindness Registry study [20]. Most of the other studies (such as the popularized COMPARE study [21]) are off-label (e.g., in standalone use) and/or include older-generation technology (i.e., iStent rather than iStent inject), so they are of limited clinical relevance to practicing surgeons. Moreover, the existing research has minimal to no comparative contralateral-eye data for the two technologies. To our knowledge, there are no contralateral studies to date that compare these two current technologies in on-label usage. The present study aims to fill this gap in the scientific literature.
Methods
Study Design
This retrospective analysis is a single-center, single-surgeon comparison of patients who underwent phacoemulsification cataract surgery combined with implantation of iStent inject or iStent inject W in the right eye or Hydrus Microstent in the left eye. In eyes with greater preoperative disease burden (characterized by one or more of the following: unmedicated IOP > 18 mmHg, treatment with two or more medications, and/or concerning visual field or optic nerve abnormalities), ABiC was completed prior to iStent inject or Hydrus implantation.
All patients were reviewed preoperatively and at 3, 6, and 12 months postoperatively. The primary outcome measures were mean IOP and mean number of glaucoma medications. Secondary outcomes included the proportions of eyes with IOP ≤ 15 mmHg, IOP ≤ 18 mmHg, and IOP reduction ≥ 20% from baseline, and on zero medications. Safety data consisted of intraoperative complications, postoperative adverse events, and secondary surgeries. The study was conducted according to the tenets of the Helsinki Declaration of 1964, local ethics guidelines (Western Institutional Review Board [IRB]), and HIPAA [Health Insurance Portability and Accountability Act] privacy practices. All patients gave informed consent prior to undergoing surgery, and their data were analyzed in a retrospective anonymized fashion.
Subject Selection
Subjects were included from a single center in this retrospective contralateral-eye analysis. All patients aged 18 years or above with a diagnosis of POAG were eligible for inclusion. Preoperatively, patients were reviewed for best-corrected visual acuity (BCVA), IOP measured with Goldman applanation tonometry, gonioscopy, slit lamp exam, and examination of the fundus and optic disc. Exclusion criteria included evidence of a comorbid ocular pathology that could confound study outcomes, and abnormalities of the anterior chamber angle such as peripheral anterior synechiae or angle recession.
Surgical Technique
All surgeries were performed by a single surgeon (MS). Phacoemulsification was performed as standard using a clear corneal temporal incision. Where applicable, ABiC was performed as described previously [22]. First, a small goniotomy was created, and then the canaloplasty catheter (either iTrack, Nova Eye Medical, Fremont, CA, USA; or OMNI Surgical System, Sight Sciences, Menlo Park, CA, USA) was advanced into Schlemm’s canal. The catheter was withdrawn while injecting viscoelastic to distend Schlemm’s canal and the orifices of collector channels. After phacoemulsification with or without canaloplasty, two iStent inject stents or one Hydrus stent were implanted in the right or left eye, respectively. The surgeries were not completed in any particular order. That is, the first operation was based on which eye the patient preferred to have done first. Then, approximately 2–3 weeks after the first surgery, surgery was completed in the contralateral eye.
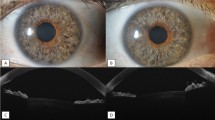
Implantation of the two iStent inject stents (either original iStent inject or wide-flange iStent inject W; Fig. 1) was completed as described previously [23]. After insertion through the temporal clear corneal incision, the injector was advanced across the anterior chamber to the nasal angle. The protective sleeve was retracted and the injector tip was used to engage the trabecular meshwork perpendicularly and lightly dimple it. The implant was then released. The steps were repeated for the second implant.
Insertion of the Hydrus device (Fig. 1) was completed using a hand-held injector through a temporal clear corneal incision as described previously [24]. The surgical microscope and the patient’s head were adjusted to allow a clear view of the nasal angle using a surgical gonioprism, and then viscoelastic was injected into the anterior chamber to distend it and expand the angle. The Microstent was introduced using the injector into the anterior chamber, and the cannula tip was used to incise the trabecular meshwork. The Hydrus Microstent was advanced through Schlemm’s canal to span three clock hours, with the 1–2 mm inlet segment remaining in the anterior chamber.
Following device implantation in either group, appropriate device positioning was confirmed, the injector was withdrawn, viscoelastic was removed, and the anterior chamber was filled with balanced salt solution. Postoperatively, patients received topical antibiotics for 10 days (besifloxacin or ofloxacin), and topical anti-inflammatory medication for 4–6 weeks (loteprednol and bromfenac).
Statistical Analysis
Statistical analysis was performed using a commercially available statistical software package (IBM SPSS Statistics for Windows, Version 20.0, released 2015. IBM Corp., Armonk, NY, USA.). Outcomes were reported as mean ± SD unless otherwise indicated. Within each group (iStent inject or Hydrus), postoperative IOP and medications versus baseline were compared using paired t tests (for continuous variables) or z tests (for categorical variables). The mean IOP and medications were compared between the two treatment groups (iStent inject versus Hydrus) using two-sample t tests. The amount of IOP and medication reduction from baseline for iStent inject versus Hydrus was compared with nonparametric tests. Subgroup analyses were completed for eyes with or without concomitant ABiC, including comparisons between the two devices (iStent inject or Hydrus), as well as between eyes with or without ABiC. A p value of < 0.05 was considered statistically significant.
Results
Demographics
A total of 40 patients (80 eyes) with mean age of 74.2 ± 8.4 years were included in this study; 69, 51, and 46 eyes had follow-up data available at 3, 6, and 12 months, respectively. Concomitant ABiC was completed in 27 eyes in the iStent inject group and 31 eyes in the Hydrus group. Table 1 shows the baseline ocular parameters of the two groups.
IOP and Medication Outcomes
The primary outcome measures, mean IOP and mean number of glaucoma medications, were measured preoperatively and at 3, 6, and 12 months. At 12 months postoperatively versus baseline, the mean IOP reduced from 16.8 ± 3.7 to 13.6 ± 2.9 (p = 0.003) in iStent inject eyes, and from 18.1 ± 4.5 to 14.9 ± 3.2 mmHg (p = 0.003) in Hydrus eyes (Table 2, Fig. 2). The mean number of glaucoma medications reduced from 1.23 ± 0.97 to 0.30 ± 0.76 (p < 0.001) in iStent inject eyes, and from 1.20 ± 1.02 to 0.39 ± 0.72 (p = 0.001) in Hydrus eyes (Table 2, Fig. 3). There was no statistically significant difference between the two devices in mean IOP pre- or postoperatively (p = 0.245 and 0.132, respectively), or in mean number of glaucoma medications pre- or postoperatively (p = 0.854 and p = 0.522, respectively). There also was no difference in the amount of IOP or medication reduction experienced by the two groups versus their respective preoperative values (between-group p = 0.582 and p = 0.943, respectively).
Mean intraocular pressure for iStent inject and Hydrus groups through 12 months. *Paired t test (Wilcoxon) for continuous variables. #Nonparametric test comparing the degree of IOP reduction from preoperative values for iStent inject vs. Hydrus. All procedures were combined with phacoemulsification cataract surgery and intraocular lens implantation. Preop preoperative, M months, IOP intraocular pressure
Mean number of medications for iStent inject and Hydrus groups through 12 months. *Paired t test (Wilcoxon) for continuous variables. #Nonparametric test comparing the degree of medication reduction from preoperative values for iStent inject vs. Hydrus. All procedures were combined with phacoemulsification cataract surgery and intraocular lens implantation. Preop preoperative, M months, Meds medications
At 12 months, the percentage of eyes with IOP ≤ 15 mmHg was 78.3% for iStent inject (vs. 40.0% preoperatively, p = 0.0076) and 52.2% for Hydrus (vs. 27.5% preoperatively, p = 0.0917). At 12 months, 82.6% of iStent inject eyes and 73.9% of Hydrus eyes were medication-free, versus 20.0% in both groups preoperatively (p < 0.0001 both groups). Table 3 and Fig. 4 summarize the proportional IOP and medication changes at baseline versus 12 months.
Preoperative vs. month 12 proportion of eyes medication-free and proportion of eyes with IOP ≤ 15 mmHg, iStent inject vs. Hydrus. *Two-proportion Z test. All procedures were combined with phacoemulsification cataract surgery and intraocular lens implantation. Preop preoperative, M months, Meds medications, IOP intraocular pressure
Comparison of iStent inject versus Hydrus, with Stratification by Ab Interno Canaloplasty
Approximately three-fourths of eyes (27 iStent inject, 31 Hydrus) underwent ABiC prior to iStent inject or Hydrus implantation, so the dataset was further stratified to compare outcomes without/with ABiC (Table 4a and b). At baseline and 12 months, in cases without ABiC, IOP reduced from 19.5 ± 2.6 to 14.8 ± 3.6 (iStent inject) and from 18.0 ± 4.9 to 16.4 ± 2.4 (Hydrus). The mean number of medications reduced from 0.69 ± 0.63 to 0.12 ± 0.35 (iStent inject) and from 0.89 ± 0.60 to 0.14 ± 0.38 (Hydrus). There were no statistically significant differences between the iStent inject and Hydrus subgroups pre- or postoperatively, either in the mean IOP and medications (Table 4a) or in the amount of reduction versus baseline (Table 4b).
In cases with concomitant ABiC, mean IOP reduced from 15.5 ± 3.5 to 12.9 ± 2.3 (iStent inject) and from 18.1 ± 4.5 to 14.3 ± 3.3 (Hydrus), mean number of medications reduced from 1.48 ± 1.01 to 0.40 ± 0.91 (iStent inject) and from 1.29 ± 1.1 to 0.50 ± 0.82 (Hydrus). No statistically significant postoperative differences existed between the two subgroups, either in the mean IOP and medications (Table 4a) or in the amount of reduction versus baseline (Table 4b).
Comparison of Eyes With or Without Ab Interno Canaloplasty for Each Device
In addition to comparing iStent inject versus Hydrus in the overall cohort and in the subgroups with or without ABiC, a separate analysis was completed to compare eyes with or without ABiC. Results are shown in Table 5 for the overall cohort and for the iStent inject and Hydrus subgroups. No statistically significant differences were observed in the amount of IOP or medication reduction experienced by the with ABiC versus without ABiC subgroups.
Adverse Events and Secondary Surgeries
No intraoperative complications occurred in either group. Postoperatively, no adverse events occurred in the iStent inject group. Postoperatively in the Hydrus group, there was one device dislocation and one device malposition. The case of dislocated Hydrus was noted at 1 week postoperatively and was accompanied by IOP of 24 mmHg (versus 20 mmHg preoperatively and 11 mmHg on day 1). The dislocation was managed with Hydrus repositioning at 1 week, followed by Hydrus exchange with goniotomy at 1 month; IOP remained ≤ 18 mmHg at study visits thereafter. The case of malposition was noted at month 1 and was accompanied by IOP elevation to 26 mmHg (versus 16 mmHg preoperatively, 13 mmHg on day 1, and 21 mmHg at week 1). The malposition required Hydrus removal with goniotomy; IOP was 17 mmHg at the subsequent visit. Based on prior experience, in both of these cases, it is likely there were areas within Schlemm’s canal that were blocked. In such areas, the viscodilation catheter could have broken through trabecular meshwork into an area of Schlemm’s canal that was not visualized by the gonioprism during catheterization, thereby creating a space for the Hydrus to inadvertently enter.
Discussion
The use of MIGS in the treatment of glaucoma has significantly changed the way glaucoma is managed. MIGS is particularly useful in those patients who are not controlled on topical treatment alone but for whom more invasive filtration surgeries are either not appropriate or present an unfavorable side effect profile. There is now a wide array of potential MIGS therapies with varying mechanisms of action. The majority of clinical studies investigating MIGS have focused on individual devices, with fewer studies of paired procedures or comparisons of different procedures [20, 21, 25, 26]. A greater understanding of how MIGS procedures can be combined, or their comparative utility, may reveal potential [27] new treatment approaches for patients who might otherwise experience disease progression requiring invasive filtration surgery. To that end, the present study supplies data on the paired use of trabecular micro-bypass and ABiC, as well as a comparison of two MIGS devices (iStent inject vs. Hydrus). The study is, to our knowledge, the first published contralateral-eye data to compare the iStent inject and Hydrus devices.
The comparative IOP and medication outcomes of the two devices in this study are consistent with prior studies of iStent or iStent inject versus Hydrus. For example, a 24-month large multicenter independent comparison study by Holmes et al. of iStent inject versus Hydrus demonstrated no statistically significant differences in IOP lowering at 24 months, and a potential additional medication reduction with iStent inject [20]. A prospective comparative study of iStent inject versus Hydrus with phacoemulsification showed similar IOP and medication reductions with the two devices [28]. A study by Lee et al. comparing iStent versus Hydrus in combination with phacoemulsification also showed no difference in IOP outcomes [29]. Overall safety profiles in these studies were generally similar or slightly more favorable for iStent or iStent inject than Hydrus, including more device-related adverse events and secondary surgeries with Hydrus [28], and more intraoperative complications such as device repositioning needed with Hydrus [29].
The present study demonstrated comparable IOP outcomes and medication reduction in both groups, suggesting that the two devices have comparable efficacy when treating glaucoma. More adverse events and additional surgeries were required in the Hydrus group, including one case requiring Hydrus repositioning and another case requiring Hydrus removal. Meanwhile, no adverse events occurred in the iStent inject group. These findings are consistent with the study by Lee et al., which reported device repositioning in 9/52 Hydrus eyes versus 0/50 iStent eyes [29]. Malposition of stents can result in significant ocular damage including corneal decompensation, uveitis, iris trauma, and pigment dispersion syndrome. Not surprisingly, in the 5-year extension study of the Hydrus pivotal trial, approximately 20% of eyes had corneal endothelial cell loss ≥ 30% versus preoperative values [30].
In addition to comparing the iStent inject and Hydrus devices, the current study contributes data on the paired use of ABiC with either device in approximately three-fourths of eyes [31, 32]. Canaloplasty can be performed as a standalone procedure or in combination with other surgeries [33, 34]. In our experience, canaloplasty can be used to open the angle, allowing easier visualization of the trabecular meshwork and facilitating MIGS placement, and it may be combined with stent implantation to further lower the IOP [35]. In the current study, although sample sizes were limited, there appeared to be a preliminary trend toward lower final IOP when pairing either device with canaloplasty compared to either device alone (Table 4a); this coincides with the recently published findings of Gallardo et al. [36]. However, given the baseline differences between subgroups, any such trends are to be interpreted with caution. Regardless of whether canaloplasty had been performed, there was still no difference in effectiveness outcomes between the two devices.
Limitations in this real-world patient cohort include loss to follow-up, yielding diminishing sample sizes especially in later months of the study. The randomization scheme was based on the laterality of the eye; however, this would not be expected to impact outcomes given there is no biological mechanism for laterality to impact IOP. Sample size was modest, and no contralateral-eye adjustments were made. Approximately three-fourths of stent surgeries were performed in combination with ABiC, thereby introducing another variable into the study; however, given that a similar percentage of eyes in either group had ABiC, the between-group comparisons would remain valid. Additionally, any potential impact of ABiC was mitigated by stratifying for whether or not ABiC was completed, and these stratified outcomes remained similar between devices. The study was designed to minimize bias, but given the surgical nature of the intervention, masking for the surgical method was not possible. Future studies could include a multicenter multi-surgeon design to avoid any potential surgical methodological bias, along with larger sample size and statistical adjustments for contralateral eyes.
Conclusion
In conclusion, this study shows comparable IOP- and medication-reducing effectiveness of iStent inject or Hydrus in combination with phacoemulsification. Effectiveness outcomes remained similar across subgroups regardless of whether concomitant ABiC had been completed. The main distinction between the devices was the different safety profile, as more complications and additional surgical procedures occurred with Hydrus than with iStent inject. These findings provide valuable real-world information to surgeons and patients who must weigh the benefits and risks of any given MIGS surgery.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tham Y-CC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34.
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97.
Bengtsson B, Heijl A. A long-term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. J Glaucoma. 2005. https://doi.org/10.1097/01.ijg.0000151683.04410.f3. (Epub ahead of print April 2005).
Leske MC, Heijl A, Hyman L, et al. Early manifest glaucoma trial: design and baseline data. Ophthalmology. 1999;106:2144–53.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311:1901.
Nordmann J-P, Auzanneau N, Ricard S, et al. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003;1:75.
Wang T, Cao L, Jiang Q, et al. Topical medication therapy for glaucoma and ocular hypertension. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.749858. (Epub ahead of print 1 December 2021).
Arbabi A, Bao X, Shalaby WS, et al. Systemic side effects of glaucoma medications. Clin Exp Optom. 2022;105:157–65.
Tappeiner C, Perren B, Iliev M, et al. Orbitale Fettgewebsatrophie bei lokaler Bimatoprost-Therapie - Kann Bimatoprost einen Enophthalmus verursachen? Klin Monbl Augenheilkd. 2008;225:443–5.
Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789-803.e2.
Salim S. Current variations of glaucoma filtration surgery. Curr Opin Ophthalmol. 2012;23:89–95.
Hirooka K, Nitta E, Ukegawa K, et al. Vision-related quality of life following glaucoma filtration surgery. BMC Ophthalmol. 2017;17:66.
Borisuth NSC, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10:112–6.
Gedde SJ, Herndon LW, Brandt JD, et al. Surgical complications in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2007;143:23-31.e2.
Pillunat LE, Erb C, Jünemann AG, et al. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–600.
Nichani P, Popovic MM, Schlenker MB, et al. Microinvasive glaucoma surgery: a review of 3476 eyes. Surv Ophthalmol. 2021;66:714–42.
Rosdahl JA, Gupta D. Prospective studies of minimally invasive glaucoma surgeries: systematic review and quality assessment. Clin Ophthalmol. 2020;14:231–43.
Birnbaum FA, Neeson C, Solá-Del VD. Microinvasive glaucoma surgery: an evidence-based review. Semin Ophthalmol. 2021;36:772–86.
Holmes DP, Clement CI, Nguyen V, et al. Comparative study of 2-year outcomes for Hydrus or iStent inject microinvasive glaucoma surgery implants with cataract surgery. Clin Exp Ophthalmol. 2022;50:303–11.
Ahmed IIK, Fea A, Au L, et al. A prospective randomized trial comparing Hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma. Ophthalmology. 2020;127:52–61.
Davids AM, Pahlitzsch M, Boeker A, et al. Ab interno canaloplasty (ABiC)—12-month results of a new minimally invasive glaucoma surgery (MIGS). Graefe’s Arch Clin Exper Ophthalmol. 2019;257:1947–53.
Samuelson TW, Sarkisian SRJ, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126:811–21.
Samet S, Ong JA, Ahmed IIK. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis. 2019;6:32.
Hu R, Guo D, Hong N, et al. Comparison of Hydrus and iStent microinvasive glaucoma surgery implants in combination with phacoemulsification for treatment of open-angle glaucoma: systematic review and network meta-analysis. BMJ Open. 2022;12: e051496.
Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: a meta-analysis. PLoS One. 2015;10: e0131770.
Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003;110:1185–91.
Stephens D. Comparison of 2 different trabecular MIGS devices in patients with mild to moderate open-angle glaucoma. Washington 2022.
Lee GA, Porter AJ, Vincent RA, et al. Combined phacoemulsification and microinvasive glaucoma surgery in comparison to phacoemulsification alone for open angle glaucoma. Eye. 2020;34:312–8.
Ahmed IIK, De Francesco T, Rhee D, et al. Long-term outcomes from the HORIZON randomized trial for a schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129:742–51.
Hughes T, Traynor M. Clinical results of Ab interno canaloplasty in patients with open-angle glaucoma. Clin Ophthalmol. 2020;14:3641–50.
Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum der Augenheilkunde. 2018;32:223–7.
Heersink M, Dovich JA. Ab interno canaloplasty combined with trabecular bypass stenting in eyes with primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1533–42.
Gołaszewska K, Konopińska J, Obuchowska I. Evaluation of the efficacy and safety of canaloplasty and iStent bypass implantation in patients with open-angle glaucoma: a review of the literature. J Clin Med. 2021;10:4881.
Shultz M. Effectiveness and safety of 2 different trabecular MIGS devices in patients with mild to moderate glaucoma. Washington, D.C.: Annual Meeting of the American Society of Cataract and Refractive Surgery (ASCRS) 2022.
Gallardo MJ, Porter M. Efficacy and safety of pairing iStent inject trabecular micro-bypass and iAccess precision blade goniotomy in patients with open-angle glaucoma. Ophthalmol Ther. 2023;12(4):1973–87.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Editorial assistance (Dana M. Hornbeak, MD, MPH and GP Communications) was provided by Glaukos Corporation.
Funding
No funding was provided for the clinical work in this postmarket retrospective analysis. The journal’s Rapid Service fee was provided by Glaukos Corporation.
Author information
Authors and Affiliations
Contributions
Mitchell Shultz: Research design, data acquisition and research execution, data analysis, data interpretation, manuscript preparation. Abraham Chorbajian: Data acquisition and research execution, data analysis, manuscript preparation. Ala Zohouralen: Data acquisition and research execution, data analysis, manuscript preparation.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Shultz: Aerie, R; Alchemy Vision, O; Allergan, C; Avellino Lab, C; Bausch and Lomb, C, R, L; BVI, R; Cloud Break, R; Glaukos, C, R, L; Glint Pharma, C; HDMD, C; HDMD, C; Imprimis/Harrow, C; Ivantis, C, L; New World Medical, C, R; Ocuphire Pharm, C; Sight Sciences, C, L; SOMED, C; SUN Pharm, C; Tarsus, R; Twenty Twenty, C; Zeiss, C. (C, consulting fees; R, research fees; L, lecture fees). Mr. Chorbajian: no competing interests. Ms. Zohouralen: no competing interests.
Ethical Approval
The study was conducted according to the tenets of the Helsinki Declaration of 1964, local ethics guidelines (Western IRB), and HIPAA privacy practices. All patients gave informed consent prior to undergoing surgery, and their data were analyzed in a retrospective anonymized fashion.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shultz, M., Chorbajian, A. & Zohouralen, A. Comparative Effectiveness and Safety of Two Different Trabecular MIGS Devices With and Without Ab Interno Canaloplasty in Patients with Primary Open-Angle Glaucoma. Ophthalmol Ther 12, 3307–3322 (2023). https://doi.org/10.1007/s40123-023-00819-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00819-5