Abstract
A single-stage anaerobic fluidized membrane bioreactor (AnFMBR) was applied to investigate the effects of temperature changes on membrane fouling while treating real municipal wastewater. The AnFMBR was operated at four temperature phases: 25 °C for 42 days, 20 °C for 20 days, 15 °C for 15 days, and at 10 °C for 15 days. The systems achieved a total chemical oxygen demand (TCOD) removal efficiency of above 90% at all phases. As temperature decreased, accumulation of solids and possible incomplete hydrolysis led to an increase in TCOD and volatile fatty acids (VFAs) in the reactor. However, as temperature reduced to 10 °C, VFAs in the reactor reduced probably an indication of reactors adaptation. Total membrane filtration resistance gradually increased to 1.1 × 1011 m−1 from 2.1 × 1009 m−1 with a temperature decrease from 25 °C to 10 °C. This corresponded to a significant decrease in membrane permeability from 1.68 to 0.05 LMH/kpa. The protein fraction of the extracellular polymeric substances (EPS) was dominant in all phases, which was ascribed for significant membrane fouling causing permeability deterioration. Microbial richness and diversity analysis using next generation Ion torrent sequencing methods revealed that Proteobacteria phylum was most dominant at 25 °C, whereas Bacteroidetes, which are responsible for releasing proteinaceous EPS, were most dominant at low temperatures (15 °C and 10 °C), contributing to severe fouling. In conclusion, decrease in temperature did not affect the treatment efficiency but resulted in gradual increase in membrane fouling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global water scarcity could be mitigated by reusing wastewater after appropriate treatment (Song et al. 2018). Anaerobic processes (AP) have drawn wide interest in treating municipal wastewater (MWW). APs are capable of mineralizing organics in the wastewater at reduced energy requirements and consequently converting it into energy. However, the retention of slow growing anaerobic biomass is a challenge in anaerobic process. In addition, the low strength, high particulate organic material fraction, and moderate biodegradability of the MWW makes anaerobic treatment in temperate climates challenging (Ozgun et al. 2013). As an alternative to conventional anaerobic processes, anaerobic membrane bioreactors (AnMBRs) are receiving attention due to their ability to effectively retain biomass inside the reactor. AnMBRs are an integration of anaerobic process and membrane separation (Lew et al. 2009; Gao et al. 2014b; Lei et al. 2018).
The integration of membranes into anaerobic MWW results in stable performance and a higher effluent quality in terms of chemical oxygen demand (COD), suspended solids (SS), and pathogen counts in comparison with conventional anaerobic processes (Ozgun et al. 2013; Song et al. 2018; Maaz et al. 2019). However, membrane fouling, loss of dissolved methane, higher susceptibility to accumulation of inhibitory substances (e.g., free ammonia and sulfate), remain major bottlenecks in the scalability of AnMBRs (Maaz et al. 2019). Of the major bottlenecks, membrane fouling is a key issue that demand high operational and maintenance costs of the system, including fouling control practices (both physical/mechanical) and frequent changes in requirements of membranes (shortened membrane lifespan)(Smith et al. 2012; Maaz et al. 2019).
Membrane fouling is mainly due to foulant deposition (cake/gel layer formation) on the membrane surface or their adsorption (pore blockage) on the membrane pore (Shahid et al. 2020). In AnMBR, foulants can be a mixture of organic and inorganic colloids, or biological–organic material like soluble microbial products (SMP), mainly bound protein-based EPS, biopolymer clusters (BPC) and microbial cells (Zhang et al. 2006; Lin et al. 2013; Aslam et al. 2017). Their deposition on the membrane surface significantly affects the cross-flow velocity, membrane filtration resistance, transmembrane pressure (TMP) and flux (Bagheri and Mirbagheri 2018; Hamedi et al. 2019; Chen et al. 2020). Several factors, such as membrane properties and characteristics, nature of the feed, sludge properties as well as hydrodynamic conditions and operational conditions, including organic loading rate (OLR), hydraulic retention rate (HRT), temperature, etc. have been identified to contribute to membrane fouling (He et al. 2005; Zhang et al. 2006; Bagheri and Mirbagheri 2018).
Temperature variations have a significant effect on bioprocesses, microbial community and membrane fouling (Mulder 1991; Lettinga et al. 2001; Arévalo et al. 2014) and are a critical factor in AnMBR operation in Nordic climates. The conversion of organic matter is affected at low temperatures due to inhibition of microbial activities (Ferrari et al. 2019) and thus reduced degradation efficiency (Lettinga et al. 2001; Cho et al. 2018). In addition, anaerobes respond differently to changes in temperatures and their unbalanced growth can result in the accumulation of transitional products (Ozgun et al. 2015). Differences in microbial composition have also been reported between summer and winter periods and have been linked to membrane fouling due to the accumulation of increased isolated cells (van den Brink et al. 2011). Likewise, a decrease in temperature causes biomass to release more extracellular polymeric substances (EPS) and soluble microbial products (SMPs), which are known to be major membrane foulants (van den Brink et al. 2011; Lin et al. 2013; Ma et al. 2013).
Temperature is thus a significant parameter in operating AnMBR in climates with temperature variability. Recently, a novel AnMBR configuration like anaerobic fluidized membrane bioreactor (AnFMBR), which uses scouring agents for fouling control has been researched to be energy efficient (Kim et al. 2011; Shin et al. 2014; Fang et al. 2015; Aslam et al. 2017) way of treating wastewater.
Several studies have investigated the performance of staged AnFMBR systems at ambient temperature (Kim et al. 2011; Bae et al. 2014; Gao et al. 2014a; Shin et al. 2014; Yoo et al. 2014). Gao et al. 2014a, b (Gao et al. 2014b) operated an integrated AnFMBR in a stepwise temperature drop from 35 to 15 °C and noted that the COD removal was low (51.1 ± 2.6%) at lower temperature in addition to accelerated fouling. However, most of the studies performed with the single stage AnFMBR have concentrated on reactors performance (Gao et al. 2014b; Aslam et al. 2017; Li et al. 2017) and the effects of fluidizing material (Aslam et al. 2014; Wu et al. 2015; Düppenbecker et al. 2017; Charfi et al. 2018). To our best knowledge, there are no such studies reported on the effects of temperature variation on membrane fouling and microbial community while treating real municipal wastewater in a single stage AnFMBR. Since relatively low temperatures (especially in Nordic climate) are crucial to the performance of biological treatment systems, assessment of a single staged AnFMBR performance at varying temperatures was the prime interest. Thus, this study was carried out to investigate the effects of temperature variations on system performance and membrane fouling in AnFMBR while treating real municipal wastewater. In addition, this study also highlighted the changes acquired in the microbial richness and diversity inside the AnFMBR systems under varying temperature regimes. The research was carried out between April and October 2019 at the Lappeenranta University of Technology, Department of Separation Science, Mikkeli.
Materials and methods
Reactor configuration and operating conditions
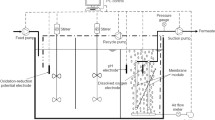
A bench-scale single stage AnFMBR reactor was constructed and employed. The AnFMBR system comprised of a plexiglass cylindrical reactor (32 mm ø and 500 mm high) with an outer water-jacketed cell for temperature control connected to a chiller and metallic settler on the top (Fig. 1). The effective volume of the reactor was about 1.5 L. A membrane module (Suez, France) made of polyvinylidene fluoride (PVDF) hollow fiber membrane (nominal pore size of 0.04 µm) with a surface area of about 0.03 m2 was used. Three peristaltic pumps (Qdos, UK) were used to feed the influent, recirculate the bulk liquid and derive permeate out of the membranes.
Granular activated carbon (GAC) was added inside the reactor to act simultaneously as an abrasive material for controlling physical membrane fouling and as an active growth support media for microorganisms. Prior to adding, the GAC was sieved through a 20 × 40 mesh to achieve a coherent size. About 50% v/v of GAC was added and fluidized by recirculating the bulk liquid from the bottom of the reactor (Fig. 1) at the flow rate of about 1 L/min, achieving a bed expansion to 100%.
Temperature, pH, and redox potential (ORP) probes were installed on the circulation line. The pH was not adjusted during this study and ranged between 6.5 and 7.8, while fluctuations in ORP values were adjusted by flushing the reactor with nitrogen gas and adjusted below −300 mV, where anaerobic conditions prevailed. The output signals from the sensors were processed by a data acquisition system (eDAQ) connected to a PC and recorded using Echem software. A pressure transducer (Omega, UK) was installed in the permeate line to measure transmembrane pressure. A Tedlar bag was connected at the top of the reactor for biogas collection.
Inoculum and substrate
The reactor was seeded with anaerobic sludge obtained from the anaerobic digester of the Kenkäveronniemi wastewater treatment plant (WWTP), Mikkeli, Finland. To ensure anaerobic conditions inside the reactor, nitrogen gas was flushed before and after the addition of inoculum. Both synthetic wastewater (SWW) and real municipal wastewater (MWW) were fed into the reactor in three different phases consecutively. In the first phase, the reactor was fed with SWW with a COD concentration of about 500 mg/L. The composition of the SWW was as follows (mg/L): glucose (C6H12O6), 143; Na-Acetate (C2H3NaO2 * 3 H2O), 321; meat peptone, 143 and urea (CH4N2O), 50. In the second phase, a 50% v/v mixture of SWW and real MWW was fed into the system. In the third phase, the reactor was fed entirely with real MWW and continued throughout the study. The COD concentration of the MWW varied between 514.0 and 782.5 mg/L during this study.
Experimental protocol
The experiment was conducted at different process temperatures, starting from 25 °C with a gradual decrease to 10 °C, to assess the effect of varying temperature regimes on membrane fouling propensity in the AnFMBR system. To ensure temperature shock on microorganisms inside the reactor, temperature changes were adjusted with the decrement rate of 5 °C/ week (van den Brink et al. 2011) to reach the desired temperature conditions before the steady-state sampling period.
The reactor was operated at 25 °C for 42 days, 20 °C for 20 days, 15 °C for 15 days, and at 10 °C for 15 days. The adopted different experimental operating conditions with the corresponding phases are summarized in Table 1.
Analytical procedures
Total chemical oxygen demand (TCOD), soluble COD (COD), ammonia nitrogen (NH3-N), ortho-phosphate (PO43−), sulfate (SO4−2), total suspended solids (TSS), volatile suspended solids (VSS), total solids (TS), total volatile solids (VS) were measured according to standard methods (APHA 1999).
Soluble microbial products (SMP) were determined by centrifuging about 35 mL of the bulk anaerobic sludge samples at 4 °C, 4000 g for 15 min. The supernatant was then filtered through a 0.45 μm glass fiber membrane. The resultant filtrate was measured as the SMP fraction. Extracellular polymeric substances (EPS) fraction was characterized into loosely bound (LB-EPS) and tightly bound (TB-EPS). To determine the LB-EPS, the retained pellets from SMP were resuspended in 30 mL of 0.5% NaCl solution and vortexed for 1 min. The settled pellets were then ultrasonicated for 5 min and centrifuged for 10 min at 4 °C, 4000 g. The supernatant was then filtered through a 0.45 μm glass fiber membrane to get the LB-EPS. Next, the retained pellet was resuspended again with 30 mL of 0.5% NaCl solution followed by 1 min vortex oscillation, 5 min ultrasonication, 30 min thermal treatment at 80 °C, centrifuged at 4 °C, 4000 g for 10 min, and finally filtered through a 0.45 μm glass fiber membrane to get tightly bound EPS (TB-EPS) for further analysis. Further, each SMP and EPS fractions were then analyzed for protein and carbohydrate contents using the spectrophotometric procedures, such as the Lowry (LOWRY et al. 1951) and Anthrone methods (Pons et al. 1981), respectively. Bovine Serum Albumin (BSA) and aqueous glucose solution were used as a standard for quantifying protein and carbohydrate fractions of SMP and EPS.
Total fouling resistance
The total membrane fouling resistance, which is the combination of membrane intrinsic resistance, cake layer resistance, and pore-blocking resistance, can be quantitatively estimated by modified Darcy’s law as given in Eq. 1 (Gurung et al. 2017):
where Rt is the total fouling resistance (m−1), TMP is the transmembrane pressure (kPa) µ is the absolute viscosity of water (Pa.s) and J is the membrane flux (LMH). Moreover, Membrane’s permeability can be calculated using Eq. (2):
The TMP values monitored online over time were used to calculate the total fouling resistance, Rt.
SEM and EDX analyses of membranes
The surface morphology and elemental composition of the pristine and fouled membrane was determined using a Hitachi S-4800 scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer (EDS). For imaging the pristine membrane surface samples were cut from the membrane before it was submerged into the reactor while the fouled membrane was collected after the operation. To prevent charge buildup on the membrane surface during SEM operation, both pristine and fouled membranes were coated with gold under vacuum.
Microbial community analysis
To investigate the influence of temperature on the AnFMBR microbial communities, biomass samples were collected in a sterile container after the reactor had reached steady-state condition at various operating temperatures. The samples (15 ml) were then centrifuged at 4000 g for 15 min at 4 °C and the settled bio-cake was collected for microbial community analysis. Prior to genomic DNA extraction, bio-cake samples were stored at −20 °C.
Genomic DNA was isolated from the bio-cake samples using a DNeasy Powersoil kit (Qiagen inc) as per manufacturer’s protocol. Approximately, 500 mg (wet weight) of bio-cake sample was used for DNA extraction. For gene libraries preparation, a fragment of the bacterial 16S rRNA gene was amplified using primers 519F (5’-CAGCMGCCCGCGGTAATWC-3’) and 926R (5’-CCGTCAATTCCTTTRAGTTT-3’). The polymerase chain reactions (PCRs) were carried out in triplicated, and the details of PCR test and associated techniques are described elsewhere (Deb et al. 2022).
Sequencing was carried out on Ion Torrent OneTouch 2 System coupled with an Ion Torrent PGM (Torrent Suite Version 5.12.0) System using Ion Hi-Q View sequencing kits (ThermoFisher Scientific, USA). The QIIME 2 platform (version 2019.7.0) was used for the analysis of sequencing data. Before loading the raw reads into QIIME 2, Cutadapt (v.2.4) was utilized to eliminate reads less than 100 bp. The DADA-2 pyro plugin was used to denoise and dereplicate readings with a truncation length of 300 bp (Callahan et al. 2016) and MAFFT was used to align the representative sequences of all distinct amplicon features (Katoh and Standley 2013). Finally, the Greengenes 13_8 99% Operational Taxonomic Unit (OTU) reference sequences were used to assign the taxonomy to all amplicon features using QIIME 2 (McDonald et al. 2012; Bokulich et al. 2018).
Results and discussion
Assessing AnFMBR system performance under varying temperature conditions
The treatment efficiency of the AnFMBR system and evolution of VFAs at different temperatures are elucidated in Table 2. The TCOD removal rate (Fig. 2) at 25 °C was 94% with an average permeate concentration of 24.74 mg/L. The removal efficiency improved to 98% with permeate quality of 15.04 mg/L. However, the TCOD removal efficiency decreased with temperature decrease to 96.10% and 94.41% while permeate concentration increased to 25.79 and 39.47 mg/L at 15 °C and 10 °C, respectively. The lower removal efficiency at startup could be attributed to the effects of the reduced temperature on the metabolism of the mesophilic anaerobic sludge used to seed the AnFMBR (Lettinga et al. 2001). However, TCOD removal efficiency remained above 90% even at lower temperatures, which may be attributed to high biomass retention by AnMBR system despite the changing temperatures (Ozgun et al. 2015). High TCOD was observed in the reactor and increased with time, which could have been a result of incomplete hydrolysis and accumulation of suspended solids (SS) (Ozgun et al. 2015). SCOD removal efficiency was 84.78% at 25 °C and 79.64% at 15 °C. The soluble organics (below 0.45 µm) can be absorbed in the membrane pores or /and by the cake layer explaining the low concentration of SCOD in permeate (Trzcinski and Stuckey 2009; Shin et al. 2014; Yoo et al. 2014).
Volatile fatty acids (VFAs) concentration in the MLSS of AnFMBR can be a measure of the system’s adaptation (Shin et al. 2014). VFAs accumulation in the reactors was observed as process temperature was reduced from 1062 mg/L at 25 °C to 4195 mg/L at 15 °C. VFAs accumulation can occur when acidogenesis occurs much faster than methanogenesis and can cause their inhibition. It is worth noting that only tiny traces of biogas generation were observed in this study. However, after a further reduction in process temperature to 10 °C the VFAs in the reactor reduced to 1240 mg/L probably indicating an adaptation by the microbes to low temperatures (Ozgun et al. 2015). A relatively low concentration of VFAs was observed in the permeate as compared to mixed liquor implying that the VFAs could be degraded by the cake layer.
There was no general trend observed for the removal of nutrients with temperature reduction. It was observed that with an average inflow concentration of 52.95 mg/L for NH+4, the concentration in the reactors increased from 59.36 mg/L at 25 °C to 185.75 mg/L at 20 °C followed by a decrease to 178 mg/L and 75.83 mg/L at 15 °C and 10 °C, respectively. The NH+4 concentration in the permeate showed a decline from 45.26 mg/L at 25 °C to 26 mg/L at 10 °C. Likewise, the concentration of total nitrogen in the reactor increased from 141,69 mg/l at 25 °C to 241.17 mg/L at 15 °C and then reduced to 174. 33 at 10 °C. Their initial increase in the reactor may be due to their high rejection by the UF membrane while the NH+4 decrease at low temperature may be due to the formation of ammonia calcium/magnesium phosphate (Chen et al. 2014). The evolution of TP indicated an increase in the reactors despite a low influent concentration ranging between 8.89 and 11.51 mg/L. The increase in TP in the reactor reached a peak of 117.50 mg/L at 15 °C from an initial of 44.81 at 25 °C, followed by a decrease to 40.40 mg/L at 10 °C. This decrease could have been due to the precipitation of phosphorus. Rejection of elements such as magnesium Mg, P, N can lead to scaling formation (Trzcinski and Stuckey 2009).
Membrane fouling assessment
Quantitative membrane fouling assessment
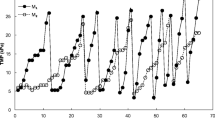
The average permeate flux was adjusted between 2 and 7 L/m2/h (LMH) during the experimental periods. Figure 2 depicts the development of total filtration resistance and permeability for the study in the four temperature phases.
The filtration resistance, which is a measure of the degree of fouling, was observed to have an increasing trend as temperature decreased, indicating that fouling was significant at low temperatures (Fig. 2). At the beginning of the experiment (25 °C), the total membrane resistance did not change distinctly since the system was conditioning during this phase and operated at low but quite stable flux. On the other hand, there was a malfunctioning of permeate pump between the end of the first phase and the beginning of the second phase (i.e., 20 °C), which interrupted the permeate flux extraction (Fig. 3).
Once the permeate pump was fixed, the average flux of 4–7 LMH was maintained, which ran until day 60 of the second phase. A gradual increase in the total fouling resistance was observed initially as process temperature was lowered to 15 °C with a significant decrease in permeability. A peak attribution of fouling was observed during this lowest temperature period about day 85 with TMP and total fouling resistance of 45 kPa and 1.1 × 1011 m−1, respectively. Severe membrane fouling at low wastewater temperature is evident in MBRs and has been reported in other studies (van den Brink et al. 2011; Gao et al. 2014b; Gurung et al. 2017). At low temperatures, the net biomass yield (g biomass g−1 substrate converted) of acidogenic sludge increases (Lettinga et al. 2001) and could result in fouling. Fouled membranes at lower temperatures have been found to have more attached biomass (Smith et al. 2013). The increased biomass can lead to the accumulation of suspended solids in the reactor, increasing their convective flow towards the membrane surface.
The membrane fouling characterization was done by analyzing the SMP and EPS in relation to their respective protein and carbohydrate fractions for each temperature phase (Fig. 4). The total SMP and EPS concentration at 25, 20, 15, 10 °C were 14.1, 16.6, 5.9, 15, 4 mg/g-MLSS and 92.2, 69.04, 60.54, 54.12 mg/g-MLSS, respectively. No general trend was observed for SMP concentration between the varying temperature phases. The concentration of carbohydrate fractions of SMP was higher than that of protein fractions at all temperatures, which is in line with the results of other researchers (van den Brink et al. 2011). The EPS concentration was reduced with a decrease in temperature. The EPS protein concentration was the predominant component at all phases and could have been due to the slow hydrolysis of proteins as compared to carbohydrates (Chen et al. 2017). The proteinaceous EPS could have played a role in increasing filtration resistance. SMP carbohydrates and EPS proteins have been reported to dominate fouled membranes in both early and late stages of fouling (Zhuo et al. 2018).
Qualitative membrane fouling assessment
The physiological structure and microanalysis of the fouled membrane surface were characterized by SEM and EDX analyses. The morphology and chemical composition of the pristine and used membranes after the experiments are shown in Fig. 5a–f.
The SEM images of the clean membrane show a smooth surface (Fig. 5b–c), while the used membrane shows a scaly and relatively rough surface (Fig. 5e–f). The reduced back transport velocity of sludge as a result of increased viscosity at low temperatures may lead to the rapid deposition of materials on the membrane surface (Martinez-Sosa et al. 2011). Similarly, biofilm developing on the membrane surface could cause pore blocking fouling and consequently contribute to cake layer build up on the membrane surface (Li and Chen 2010).
The EDX elemental analyses (Fig. 6) of the virgin membrane surface confirmed that carbon (C), oxygen (O), and fluorine (F) were the main elements detected that agree with the elemental chemical composition of the commercial PVDF membrane. The surface layer of the fouled membrane indicated the presence of C, O, F, N, Na+, Mg 2+, Al3+, Si, P, S, Ca2+ Fe3+, Cu 2+ and Zn 2+. Also, the intrinsic C, O and F contents in the fouled membrane were changed from those on the unused membrane surface. This may be attributed to the interaction of the biopolymer anion groups such like SO42−, CO32−, PO43− and OH−, and the cations as Si4+, Mg2+, Al3+, Fe3+ and Ca2+, leading to precipitation (Chen et al. 2015). Although their relative content was small coupled with the organic foulant, inorganic materials can enhance fouling (Meng et al. 2007).
Microbial community assessment under different temperatures
Microbial species richness and diversity in AnMBR are known to be affected by the operating temperature (Gao 2011). The diversity and richness of the microbial community was determined by applying the next generation Ion torrent sequencing method to the collected samples at different temperatures. The goodness of coverage for all the samples was more than 0.99, indicating that the Ion torrent sequencing was able to unveil most of the OTUs (Chen 2017). The diversity and richness of the microbial communities in the samples were characterized by the Shannon diversity index and Chao1 estimation, respectively, and the results are presented in Table 3. Both the Shannon and Chao1 index have increased with the reduction in operating temperatures.
Microbial community analysis showed that anaerobic sludge was enriched with a core group of bacterial phyla comprised of Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes. However, the relative loads and dominance of these bacteria were affected by temperature variation. For example, as shown in Fig. 7, bacteria from the Proteobacteria phylum were most dominant at temperatures 25 °C and 20 °C and their relative abundances were 42.8% and 36.9%, respectively.
On the other hand, Bacteroidetes were detected as the most dominant phyla at temperatures 15 °C and 10 °C, while their relative loadings were 28.5% and 47.9%, respectively.
Proteobacteria and firmicutes are the most available microbiota in the anaerobic system and consist of various functional bacterial species. Among the four major classes of Proteobacteria (α-, β-, γ- and δ- Proteobacteria), α-proteobacteria were the dominant class of microbial species at temperatures 25 and 20 °C. Several functional bacterial genera of Proteobacteria phylum have been identified in the mixed liquor of anaerobic sludge. For example, Acidovorax, Novosphingobium and Paracoccus are known as responsible for denitrification, Aquabacterium and Zoogloea produces EPS and helps in floc formation, Geobacter acts as Fe (III) reducing bacteria and Dechloromonas helps in phosphorus removal during anaerobic treatment (Rosselló-Mora et al. 1995; Baker et al. 1998; Lee et al. 2012; Gonzalez-Martinez et al. 2016; Roy et al. 2018). There were no clear trends of bacterial dynamics in the mixed liquor during the temporal variation; however, Novosphingobium (7.97%) were the most dominant genera at 20 °C.
In the Firmicutes phylum, Anaerovorax, Blautia, Fusibacter, Proteiniclasticum, PSB-M-3 and Streptococcus were placed in the top 30 dominant genera presented in Fig. 8. Most of the bacterial genera of Firmicutes are gram-positive and collectively play a significant role in anaerobic digestion. Anaerovorax, Blautia, Fusibacter and Proteiniclasticum belong to the class Clostridia, while Streptococcus and PSB-M-3 originated from the class Bacilli and Erysipelotrichi, respectively. Fusibacter sp. has been reported to produce acetate and butyrate from various carbohydrate molecules in the wastewater (Ravot et al. 1999). Apart from that, Anaerovorax and Proteiniclasticum have been found to contribute to biofilm formation (Liu et al. 2016). Firmicutes have been shown to accelerate biofouling in AnMBRs (Aslam et al. 2018).
The Actinobacteria phylum consists of various gram-positive aerobic and anaerobic bacterial communities and their role in sludge bulking and foaming as well as in enhanced biological phosphorus removal has been identified (Seviour et al. 2008). Actinomyces, Leucobacter, Micrococcus, Nocardioides, Phycicoccus, Propionibacterium and Rhodococcus were the major identified genera of Actinobacteria phylum. Among them, Actinomyces and Propionibacterium are known to be responsible for urea hydrolysis, ammonia release and propionic acid production (Balamurugan et al. 1999; Yaling et al. 2008). From the experimental observation, it was found that the relative abundances of Actinomyces and Propionibacterium reduced from 1.06% to 0.04% and 4.69% to 0.01%, respectively, with a reduction in temperature from 25 to 10 °C. Among other genera of Actinobacteria phylum, Micrococcus and Rhodococcus are responsible for phosphate solubilization and desulfurization, respectively (Finnerty et al. 1983; Kalayu 2019). The relative abundance of Micrococcus was 2.97% at 25 °C and further vanished when the process operating temperature reached 10 °C. However, the relative abundances of Rhodococcus bacterial species did not show a clear trend with a temporal variation.
Microbial communities from the Bacteroidetes phylum are gram-negative and known to be responsible for hydrolysis and fermentation of complex organic matter to produce VFAs, such as acetate and propionate. Their increased abundance at low temperatures might have resulted in the increased VFAs concentration in the reactor thus inhibiting methane production. Seven dominant bacterial genera were detected from the Bacteroidetes phylum which includes Chryseobacterium, Cloacibacterium, Flavobacterium, Macellibacteroides, Paludibacter, Pedobacter and Prevotella. It can also be noted that the growth of Bacteroidetes increased with temperature decline in the AnMBR. Bacteroidetes have been known to contribute to fouling through the release of proteinaceous EPS. In addition, Bacteroidetes possess fimbriae which helps them to attach to surfaces which could result in bacteria enrichment and adherence to the membrane surface and thus increased fouling at lower temperatures (Ding et al. 2019). Flavobacterium and Paludibacter showed the most increasing trend in their abundances with decreasing temperature and became the most dominant genera in the anaerobic sludge at 10 °C with a relative abundance of 7.4% and 6.1%, respectively. Paludibacters are chemoorganotrophic and utilize glucose and soluble starch as a substrate for their growth (Gronow et al. 2011). Among other genera, Chryseobacterium and Pedobacter have been reported as antibiotic resistant bacteria (Fraser and Jorgensen 1997; Viana et al. 2018). Maximum abundance of Chryseobacterium was obtained at 15 °C.
Conclusion
A single-stage AnFMBR was operated continuously at four different temperature phases: 25 °C for 42 days, 20 °C for 20 days, 15 °C for 15 days, and at 10 °C for 15 days, respectively. The systems achieved a total chemical oxygen demand (TCOD) removal efficiency of above 90% at all phases, despite temperature changes. Accumulation of solids and incomplete hydrolysis led to an increase in reactors TCOD and VFAs as temperature decreased. Total membrane filtration resistance gradually increased as the temperature decreased to 15 °C with a significant decrease in membrae permeability. The protein fraction of the EPS was dominant in all phases, which was ascribed to significant membrane fouling, thereby causing permeability deterioration. The SEM–EDX analysis of the fouled membrane after the completion of the experiments showed substantial deposits on the membrane surface with a scaling layer of organic and inorganic substances. Moreover, microbial richness and diversity analysis revealed Proteobacteria phylum as the most dominant at 25 °C, whereas, Bacteroidetes, which are meant for releasing proteinaceous EPS, were most dominant at 15 °C and 10 °C, contributing to severe fouling.
The result of this study shows that AnFMBRs can be used for the effective treatment of domestic wastewater. Biogas production in this case was challenging and thus should be further researched to make the system energy efficient. Further studies should focus into long term operations with lower temperatures and investigation on the development of attachment growth of microorganisms responsible for releasing proteinaceous EPS, the effects of GAC dosage in elevating fouling. In addition, future consideration should be placed on ways of enhancing macronutrient removal and recovery in this type of reactor.
References
Arévalo J, Ruiz LM, Pérez J, Gómez MA (2014) Effect of temperature on membrane bioreactor performance working with high hydraulic and sludge retention time. Biochem Eng J. https://doi.org/10.1016/j.bej.2014.03.006
Aslam M, McCarty PL, Bae J, Kim J (2014) The effect of fluidized media characteristics on membrane fouling and energy consumption in anaerobic fluidized membrane bioreactors. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2014.04.049
Aslam M, McCarty PL, Shin C et al (2017) Low energy single-staged anaerobic fluidized bed ceramic membrane bioreactor (AFCMBR) for wastewater treatment. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.03.017
Aslam M, Yang P, Lee PH, Kim J (2018) Novel staged anaerobic fluidized bed ceramic membrane bioreactor: energy reduction, fouling control and microbial characterization. J Memb Sci. https://doi.org/10.1016/j.memsci.2018.02.038
Bae J, Shin C, Lee E et al (2014) Anaerobic treatment of low-strength wastewater: a comparison between single and staged anaerobic fluidized bed membrane bioreactors. Bioresour Technol. https://doi.org/10.1016/j.biortech.2014.02.065
Bagheri M, Mirbagheri SA (2018) Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour Technol 258
Baker SC, Ferguson SJ, Ludwig B et al (1998) Molecular genetics of the GenusParacoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev 62:1046–1078. https://doi.org/10.1128/MMBR.62.4.1046-1078.1998
Balamurugan K, Venkata Dasu V, Panda T (1999) Propionic acid production by whole cells of Propionibacterium freudenreichii. Bioprocess Eng 20:109–116. https://doi.org/10.1007/PL00009039
Bokulich NA, Kaehler BD, Rideout JR et al (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. https://doi.org/10.1186/s40168-018-0470-z
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Charfi A, Park E, Aslam M, Kim J (2018) Particle-sparged anaerobic membrane bioreactor with fluidized polyethylene terephthalate beads for domestic wastewater treatment: modelling approach and fouling control. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.02.093
Chen C, Mao S, Wang J et al (2015) Application of ultrafiltration in a paper mill: process water reuse and membrane fouling analysis. BioResources. https://doi.org/10.15376/biores.10.2.2376-2391
Chen L, Cheng P, Ye L et al (2020) Biological performance and fouling mitigation in the biochar-amended anaerobic membrane bioreactor (AnMBR) treating pharmaceutical wastewater. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.122805
Chen L, Gu Y, Cao C et al (2014) Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. https://doi.org/10.1016/j.watres.2013.12.009
Chen R, Nie Y, Hu Y et al (2017) Fouling behaviour of soluble microbial products and extracellular polymeric substances in a submerged anaerobic membrane bioreactor treating low-strength wastewater at room temperature. J Memb Sci. https://doi.org/10.1016/j.memsci.2017.02.046
Cho K, Jeong Y, Seo KW et al (2018) Effects of changes in temperature on treatment performance and energy recovery at mainstream anaerobic ceramic membrane bioreactor for food waste recycling wastewater treatment. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.02.015
Deb A, Gurung K, Rumky J et al (2022) Dynamics of microbial community and their effects on membrane fouling in an anoxic-oxic gravity-driven membrane bioreactor under varying solid retention time: a pilot-scale study. Sci Total Environ 807:150878. https://doi.org/10.1016/j.scitotenv.2021.150878
Ding Y, Liang Z, Guo Z et al (2019) The performance and microbial community identification in mesophilic and atmospheric anaerobic membrane bioreactor for municipal wastewater treatment associated with different hydraulic retention times. Water (switzerland). https://doi.org/10.3390/w11010160
Düppenbecker B, Engelhart M, Cornel P (2017) Fouling mitigation in anaerobic membrane bioreactor using fluidized glass beads: evaluation fitness for purpose of ceramic membranes. J Memb Sci. https://doi.org/10.1016/j.memsci.2017.05.018
Fang HHP, Zhang T, McCarty PL, et al (2015) Anaerobic fluidized bed membrane bioreactors for the treatment of domestic wastewater. In: Anaerobic biotechnology
Ferrari F, Balcazar JL, Rodriguez-Roda I, Pijuan M (2019) Anaerobic membrane bioreactor for biogas production from concentrated sewage produced during sewer mining. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.03.218
Finnerty WR, Shockley K, Attaway H (1983) Microbial desulfurization and denitrogenation of hydrocarbons. Microb Enhanc Oil Recover, pp 83–91
Fraser SL, Jorgensen JH (1997) Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother 41:2738–2741. https://doi.org/10.1128/AAC.41.12.2738
Gao DW, Hu Q, Yao C et al (2014a) Integrated anaerobic fluidized-bed membrane bioreactor for domestic wastewater treatment. Chem Eng J. https://doi.org/10.1016/j.cej.2013.12.012
Gao DW, Hu Q, Yao C, Ren NQ (2014b) Treatment of domestic wastewater by an integrated anaerobic fluidized-bed membrane bioreactor under moderate to low temperature conditions. Bioresour Technol. https://doi.org/10.1016/j.biortech.2014.02.086
Gonzalez-Martinez A, Rodriguez-Sanchez A, Lotti T et al (2016) Comparison of bacterial communities of conventional and A-stage activated sludge systems. Sci Rep 6:18786. https://doi.org/10.1038/srep18786
Gronow S, Munk C, Lapidus A et al (2011) Complete genome sequence of Paludibacter propionicigenes type strain (WB4T). Stand Genomic Sci 4:36–44. https://doi.org/10.4056/sigs.1503846
Gurung K, Ncibi MC, Sillanpää M (2017) Assessing membrane fouling and the performance of pilot-scale membrane bioreactor (MBR) to treat real municipal wastewater during winter season in Nordic regions. Sci Total Environ 579:1289–1297
Hamedi H, Ehteshami M, Mirbagheri SA, et al (2019) Current status and future prospects of membrane bioreactors (MBRs) and fouling phenomena: a systematic review. Can. J Chem Eng, 97
He Y, Xu P, Li C, Zhang B (2005) High-concentration food wastewater treatment by an anaerobic membrane bioreactor. Water Res. https://doi.org/10.1016/j.watres.2005.07.030
Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int J Agron 2019:1–7. https://doi.org/10.1155/2019/4917256
Katoh K, Standley DM (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kim J, Kim K, Ye H et al (2011) Anaerobic fluidized bed membrane bioreactor for wastewater treatment. Environ Sci Technol. https://doi.org/10.1021/es1027103
Lee SH, Jin HM, Lee HJ et al (2012) Complete genome sequence of the BTEX-degrading bacterium pseudoxanthomonas spadix BD-a59. J Bacteriol 194:544–544. https://doi.org/10.1128/JB.06436-11
Lei Z, Yang S, Li Y et al (2018) Application of anaerobic membrane bioreactors to municipal wastewater treatment at ambient temperature: a review of achievements, challenges, and perspectives. Bioresour Technol. https://doi.org/10.1016/j.biortech.2018.07.050
Lettinga G, Rebac S, Zeeman G (2001) Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol 19:363–370
Lew B, Tarre S, Beliavski M et al (2009) Anaerobic membrane bioreactor (AnMBR) for domestic wastewater treatment. Desalination. https://doi.org/10.1016/j.desal.2008.04.027
Li H, Chen V (2010) Membrane fouling and cleaning in food and bioprocessing. In: Membrane technology
Li Y, Hu Q, Chen CH et al (2017) Performance and microbial community structure in an integrated anaerobic fluidized-bed membrane bioreactor treating synthetic benzothiazole contaminated wastewater. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.03.189
Lin H, Peng W, Zhang M, et al (2013) A review on anaerobic membrane bioreactors: applications, membrane fouling and future perspectives. Desalination 314
Liu W, He Z, Yang C et al (2016) Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol Biofuels 9:83. https://doi.org/10.1186/s13068-016-0493-2
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. https://doi.org/10.1016/0922-338X(96)89160-4
Ma Z, Wen X, Zhao F et al (2013) Effect of temperature variation on membrane fouling and microbial community structure in membrane bioreactor. Bioresour Technol. https://doi.org/10.1016/j.biortech.2013.01.023
Maaz M, Yasin M, Aslam M, et al (2019) Anaerobic membrane bioreactors for wastewater treatment: novel configurations, fouling control and energy considerations. Bioresour Technol, 283
Martinez-Sosa D, Helmreich B, Netter T et al (2011) Anaerobic submerged membrane bioreactor (AnSMBR) for municipal wastewater treatment under mesophilic and psychrophilic temperature conditions. Bioresour Technol. https://doi.org/10.1016/j.biortech.2011.09.012
McDonald D, Price MN, Goodrich J et al (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618. https://doi.org/10.1038/ismej.2011.139
Meng F, Zhang H, Yang F, Liu L (2007) Characterization of cake layer in submerged membrane bioreactor. Environ Sci Technol. https://doi.org/10.1021/es062208b
Mulder M (1991) Basic principles of membrane technology. https://doi.org/10.1524/zpch.1998.203.part_1_2.263
Ozgun H, Dereli RK, Ersahin ME, et al (2013) A review of anaerobic membrane bioreactors for municipal wastewater treatment: integration options, limitations and expectations. Sep Purif Technol, 118
Ozgun H, Tao Y, Ersahin ME et al (2015) Impact of temperature on feed-flow characteristics and filtration performance of an upflow anaerobic sludge blanket coupled ultrafiltration membrane treating municipal wastewater. Water Res. https://doi.org/10.1016/j.watres.2015.06.035
Pons A, Roca P, Aguiló C et al (1981) A method for the simultaneous determinations of total carbohydrate and glycerol in biological samples with the anthrone reagent. J Biochem Biophys Methods. https://doi.org/10.1016/0165-022X(81)90060-9
Ravot G, Magot M, Fardeau M-L et al (1999) Fusibacter paucivorans gen. nov., sp. nov., an anaerobic, thiosulfate-reducing bacterium from an oil-producing well. Int J Syst Evol Microbiol 49:1141–1147. https://doi.org/10.1099/00207713-49-3-1141
Rosselló-Mora RA, Wagner M, Amann R, Schleifer K-H (1995) The abundance of Zoogloea ramigera in sewage treatment plants. Appl Environ Microbiol 61:702–707
Roy A, Sar P, Sarkar J et al (2018) Petroleum hydrocarbon rich oil refinery sludge of North-East India harbours anaerobic, fermentative, sulfate-reducing, syntrophic and methanogenic microbial populations. BMC Microbiol 18:151. https://doi.org/10.1186/s12866-018-1275-8
Seviour RJ, Kragelund C, Kong Y et al (2008) Ecophysiology of the Actinobacteria in activated sludge systems. Antonie van Leeuwenhoek. Int J Gen Mol Microbiol 94:21–33. https://doi.org/10.1007/s10482-008-9226-2
Shahid MK, Kashif A, Rout PR, et al (2020) A brief review of anaerobic membrane bioreactors emphasizing recent advancements, fouling issues and future perspectives. J Environ Manage, 270
Shin C, McCarty PL, Kim J, Bae J (2014) Pilot-scale temperate-climate treatment of domestic wastewater with a staged anaerobic fluidized membrane bioreactor (SAF-MBR). Bioresour Technol. https://doi.org/10.1016/j.biortech.2014.02.060
Smith AL, Skerlos SJ, Raskin L (2013) Psychrophilic anaerobic membrane bioreactor treatment of domestic wastewater. Water Res. https://doi.org/10.1016/j.watres.2012.12.028
Smith AL, Stadler LB, Love NG et al (2012) Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: a critical review. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.04.055
Song X, Luo W, Hai FI, et al (2018) Resource recovery from wastewater by anaerobic membrane bioreactors: opportunities and challenges. Bioresour Technol 270
Trzcinski AP, Stuckey DC (2009) Continuous treatment of the organic fraction of municipal solid waste in an anaerobic two-stage membrane process with liquid recycle. Water Res. https://doi.org/10.1016/j.watres.2009.03.030
van den Brink P, Satpradit OA, van Bentem A et al (2011) Effect of temperature shocks on membrane fouling in membrane bioreactors. Water Res. https://doi.org/10.1016/j.watres.2011.05.046
Viana AT, Caetano T, Covas C et al (2018) Environmental superbugs: the case study of Pedobacter spp. Environ Pollut 241:1048–1055. https://doi.org/10.1016/j.envpol.2018.06.047
Wu B, Wong PCY, Fane AG (2015) The potential roles of granular activated carbon in anaerobic fluidized membrane bioreactors: effect on membrane fouling and membrane integrity. Desalin Water Treat. https://doi.org/10.1080/19443994.2014.943057
Yaling L, Dan J, Tao H, Xuedong Z (2008) Regulation of urease expression of Actinomyces naeslundii in biofilms in response to pH and carbohydrate. Oral Microbiol Immunol 23:315–319. https://doi.org/10.1111/j.1399-302X.2008.00430.x
Yoo RH, Kim JH, McCarty PL, Bae JH (2014) Effect of temperature on the treatment of domestic wastewater with a staged anaerobic fluidized membrane bioreactor. Water Sci Technol. https://doi.org/10.2166/wst.2013.793
Zhang J, Chua HC, Zhou J, Fane AG (2006) Factors affecting the membrane performance in submerged membrane bioreactors. J Memb Sci. https://doi.org/10.1016/j.memsci.2006.06.022
Zhuo M, Abass OK, Zhang K (2018) New insights into the treatment of real: N,N-dimethylacetamide contaminated wastewater using a membrane bioreactor and its membrane fouling implications. RSC Adv. https://doi.org/10.1039/c8ra01657g
Acknowledgements
The Authors would like to acknowledge the Regional Council of South Savo (Etelä-Savon Maakuntaliitto, Finland) for funding this research (Decision number; EURA 2014/7154/09 02/01/2018/ESAVO); Reijo Turkki, Anne Bergman, and Risto Repo and all other staffs of Mikkeli Wastewater Treatment Plant, Finland, on their continuous support for wastewater sampling services. In addition the authors thank the Lappeenranta University of Technology, Department of Separation Science, Mikkeli for providing the research facilities and the Biocenter Oulu for their support in microbial community analysis.
Author information
Authors and Affiliations
Contributions
Puhakka Ville was involved in conceptualization, methodology and investigation. Theuri Shelmith was involved in data curation, formal analysis and visualization. Gurung Khum was involved in validation and supervision. Theuri Shelmith and Deb Anjan were involved in writing—original draft preparation. Sillanpää Mika was involved in funding acquisition and collected resources. Gurung Khum and Sillanpää Mika were involved in writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Theuri, S., Gurung, K., Puhakka, V. et al. Effect of temperature variations in anaerobic fluidized membrane bioreactor: membrane fouling and microbial community dynamics assessment. Int. J. Environ. Sci. Technol. 20, 9451–9464 (2023). https://doi.org/10.1007/s13762-022-04648-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04648-0