Abstract
Purpose
Tumor metastasis significantly impacts the prognosis of non-small cell lung cancer (NSCLC) patients, with lymph node (LN) metastasis being the most common and early form of spread. With the development of adjuvant immunotherapy, increasing attention has been paid to the tumor-draining lymph nodes(TDLN) in early-stage NSCLC, especially tumor-metastatic lymph nodes, which provides poor prognostic information but has potential benefits in adjuvant treatment.
Methods
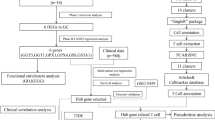
We showed the remodeled immune environment in TDLNs through using TCR-seq to analyse 24 primary lung cancer tissues and 134 LNs from 24 lung cancer patients with or without LN metastasis. Additionally, we characterized the spatial profiling of immunocytes and tumor cells in TDLNs and primary tumor sites through using multi-IHC.
Results
We found the remodeled immune environment in TDLNs through analyzing primary lung cancer tissues and LNs from NSCLC patients with or without LN metastasis. Considering the intricate communication between tumor and immunocytes, we further subdivided TDLNs, revealing that metastasis-negative LNs from LN-metastatic patients (MNLN) exhibited greater immune activation, exhaustion, and memory in comparison to both metastasis-positive LNs (MPLN) and TDLNs from non-LN-metastatic patients (NMLN).
Conclusions
Our data indicate that LN metastasis facilitated tumor-specific antigen presentation in TDLNs and induces T cell priming, while existing tumor cells generate an immune-suppressive environment in MPLNs through multiple mechanisms. These findings contribute to a comprehensive understanding of the immunological mechanisms through which LN metastasis influences tumor progression and plays a role in immunotherapy for NSCLC patients.






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
F.R. Hirsch, G.V. Scagliotti, J.L. Mulshine, R. Kwon, W.J. Curran Jr., Y.L. Wu, L. Paz-Ares, Lung cancer: current therapies and new targeted treatments. Lancet 389(10066), 299–311 (2017)
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 11(1):39–51 (2016)
M.J. O’Melia, M.P. Manspeaker, S.N. Thomas, Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer. Immunol. Immunother. 70(8), 2179–2195 (2021)
V. Murthy, J. Minehart, D. Sterman, Local immunotherapy of cancer: innovative approaches to harnessing tumor-specific immune responses. J. Natl. Cancer. Inst. 109(12), 117–129 (2017)
U.H. von Andrian, T.R. Mempel, Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3(11), 867–878 (2003)
J.H. Lee, H. Torisu-Itakara, A.J. Cochran, A. Kadison, Y. Huynh, D.L. Morton, R. Essner, Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin. Cancer Res. 11(1), 107–112 (2005)
A.S. Mansfield, S.G. Holtan, T.E. Grotz, J.B. Allred, J.W. Jakub, L.A. Erickson, S.N. Markovic, Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod. Pathol. 24(4), 487–494 (2011)
K.M. van Pul, R.J.C.L.M. Vuylsteke, R. van de Ven, E.A. Te Velde, E.J.T. Rutgers, P.M. van den Tol, H.B.A.C. Stockmann, T.D. de Gruijl, Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J. Immunother. Cancer 7(1), 133 (2019)
K. Matsuura, Y. Yamaguchi, H. Ueno, A. Osaki, K. Arihiro, T. Toge, Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 106(6), 1227–1236 (2006)
M.F.C.M. van den Hout, B.D. Koster, B.J.R. Sluijter, B.G. Molenkamp, R. van de Ven, A.J.M. van den Eertwegh, R.J. Scheper, P.A.M. van Leeuwen, M.P. van den Tol, T.D. de Gruijl, Melanoma sequentially suppresses different DC subsets in the sentinel lymph node, affecting disease spread and recurrence. Cancer Immunol. Res. 5(11), 969–977 (2017)
L. Deng, H. Zhang, Y. Luan, J. Zhang, Q. Xing, S. Dong, X. Wu, M. Liu, S. Wang, Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin. Cancer Res. 16(16), 4105–4112 (2010)
G. Li, D. Liu, T.K. Cooper, E.T. Kimchi, X. Qi, D.M. Avella, N. Li, Q.X. Yang, M. Kester, C.B. Rountree, J.T. Kaifi, D.J. Cole, D.C. Rockey, T.D. Schell, K.F. Staveley-O’Carroll, Successful chemoimmunotherapy against hepatocellular cancer in a novel murine model. J. Hepatol. 66(1), 75–85 (2017)
N.G. Núñez, J. Tosello Boari, R.N. Ramos, W. Richer, N. Cagnard, C.D. Anderfuhren, L.L. Niborski, J. Bigot, D. Meseure, P. De La Rochere, M. Milder, S. Viel, D. Loirat, L. Pérol, A. Vincent-Salomon, X. Sastre-Garau, B. Burkhard, C. Sedlik, O. Lantz, S. Amigorena, E. Piaggio, Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat. Commun. 11(1), 3272 (2020)
S.P.L. Saw, B.H. Ong, K.L.M. Chua, A. Takano, D.S.W. Tan, Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol. 22(11), e501–e516 (2021)
J.E. Chaft, Y. Shyr, B. Sepesi, P.M. Forde, Preoperative and postoperative systemic therapy for operable non-small-cell lung cancer. J. Clin. Oncol. 40(6), 546–555 (2022). https://doi.org/10.1200/JCO.21.01589
Y. Ling, N. Li, L. Li, C. Guo, J. Wei, P. Yuan, F. Tan, X. Tao, S. Wang, Z. Wang, N. Wu, J. Wang, J. Ying, S. Gao, J. He, Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis. Oncol. 4(1), 32 (2020)
A.K. Molodtsov, N. Khatwani, J.L. Vella, K.A. Lewis, Y. Zhao, J. Han, D.E. Sullivan, T.G. Searles, N.K. Preiss, T.B. Shabaneh, P. Zhang, A.R. Hawkes, B.T. Malik, F.W. Kolling 4th., E.J. Usherwood, S.L. Wong, J.D. Phillips, K. Shirai, C.V. Angeles, S. Yan, T.J. Curiel, Y.H. Huang, C. Cheng, M.J. Turk, Resident memory CD8+ T cells in regional lymph nodes mediate immunity to metastatic melanoma. Immunity 54(9), 2117-2132.e7 (2021)
J.M. Schenkel, R.H. Herbst, D. Canner, A. Li, M. Hillman, S.L. Shanahan, G. Gibbons, O.C. Smith, J.Y. Kim, P. Westcott, W.L. Hwang, W.A. Freed-Pastor, G. Eng, M.S. Cuoco, P. Rogers, J.K. Park, M.L. Burger, O. Rozenblatt-Rosen, L. Cong, K.E. Pauken, A. Regev, T. Jacks, Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity 54(10), 2338-2353.e6 (2021)
K.A. Connolly, M. Kuchroo, A. Venkat, A. Khatun, J. Wang, I. William, N.I. Hornick, B.L. Fitzgerald, M. Damo, M.Y. Kasmani, C. Cui, E. Fagerberg, I. Monroy, A. Hutchins, J.F. Cheung, G.G. Foster, D.L. Mariuzza, M. Nader, H. Zhao, W. Cui, S. Krishnaswamy, N.S. Joshi, A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 6(64), eabg7836 (2021)
M. Binnewies, A.M. Mujal, J.L. Pollack, A.J. Combes, E.A. Hardison, K.C. Barry, J. Tsui, M.K. Ruhland, K. Kersten, M.A. Abushawish, M. Spasic, J.P. Giurintano, V. Chan, A.I. Daud, P. Ha, C.J. Ye, E.W. Roberts, M.F. Krummel, Unleashing Type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell 177(3), 556-571.e16 (2019)
A.W. Lund, F.V. Duraes, S. Hirosue, V.R. Raghavan, C. Nembrini, S.N. Thomas, A. Issa, S. Hugues, M.A. Swartz, VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 1(3), 191–199 (2012)
R.D. Leone, J.D. Powell, Metabolism of immune cells in cancer. Nat. Rev. Cancer 20(9), 516–531 (2020)
N.E. Reticker-Flynn, W. Zhang, J.A. Belk, P.A. Basto, N.K. Escalante, G.O.W. Pilarowski, A. Bejnood, M.M. Martins, J.A. Kenkel, I.L. Linde, S. Bagchi, R. Yuan, S. Chang, M.H. Spitzer, Y. Carmi, J. Cheng, L.L. Tolentino, O. Choi, N. Wu, C.S. Kong, A.J. Gentles, J.B. Sunwoo, A.T. Satpathy, S.K. Plevritis, E.G. Engleman, Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 185(11), 1924-1942.e23 (2022)
N. Prokhnevska, M.A. Cardenas, R.M. Valanparambil et al., CD8(+) T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity 56, 107-124.e105 (2023)
S.P. Leong, M. Zuber, R.L. Ferris, Y. Kitagawa, R. Cabanas, C. Levenback, M. Faries, S. Saha, Impact of nodal status and tumor burden in sentinel lymph nodes on the clinical outcomes of cancer patients. J. Surg. Oncol. 103(6), 518–530 (2011)
F. Dammeijer, M. van Gulijk, E.E. Mulder, M. Lukkes, L. Klaase, T. van den Bosch, M. van Nimwegen, S.P. Lau, K. Latupeirissa, S. Schetters, Y. van Kooyk, L. Boon, A. Moyaart, Y.M. Mueller, P.D. Katsikis, A.M. Eggermont, H. Vroman, R. Stadhouders, R.W. Hendriks, J.V. Thüsen, D.J. Grünhagen, C. Verhoef, T. van Hall, J.G. Aerts, The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell 38(5), 685-700.e8 (2020)
M.H. Spitzer, Y. Carmi, N.E. Reticker-Flynn, S.S. Kwek, D. Madhireddy, M.M. Martins, P.F. Gherardini, T.R. Prestwood, J. Chabon, S.C. Bendall, L. Fong, G.P. Nolan, E.G. Engleman, Systemic immunity is required for effective cancer immunotherapy. Cell 168(3), 487-502.e15 (2017)
M.F. Fransen, M. Schoonderwoerd, P. Knopf, M.G. Camps, L.J. Hawinkels, M. Kneilling, T. van Hall, F. Ossendorp, Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 3(23), e124507 (2018)
M. Provencio, E. Nadal, A. Insa, M.R. García-Campelo, J. Casal-Rubio, M. Dómine, M. Majem, D. Rodríguez-Abreu, A. Martínez-Martí, C.J. De Castro, M. Cobo, G. López Vivanco, E. Del Barco, R. Bernabé Caro, N. Viñolas, I. Barneto Aranda, S. Viteri, E. Pereira, A. Royuela, M. Casarrubios, C. Salas Antón, E.R. Parra, I. Wistuba, V. Calvo, R. Laza-Briviesca, A. Romero, B. Massuti, A. Cruz-Bermúdez, Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 21(11), 1413–1422 (2020)
K.E. Yost, A.T. Satpathy, D.K. Wells, Y. Qi, C. Wang, R. Kageyama, K.L. McNamara, J.M. Granja, K.Y. Sarin, R.A. Brown, R.K. Gupta, C. Curtis, S.L. Bucktrout, M.M. Davis, A.L.S. Chang, H.Y. Chang, Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25(8), 1251–1259 (2019)
Z. Ding, Q. Li, R. Zhang, L. Xie, Y. Shu, S. Gao, P. Wang, X. Su, Y. Qin, Y. Wang, J. Fang, Z. Zhu, X. Xia, G. Wei, H. Wang, H. Qian, X. Guo, Z. Gao, Y. Wang, Y. Wei, Q. Xu, H. Xu, L. Yang, Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct. Target Ther. 6(1), 26 (2021)
P.A. Ott, Z. Hu, D.B. Keskin, S.A. Shukla, J. Sun, D.J. Bozym, W. Zhang, A. Luoma, A. Giobbie-Hurder, L. Peter, C. Chen, O. Olive, T.A. Carter, S. Li, D.J. Lieb, T. Eisenhaure, E. Gjini, J. Stevens, W.J. Lane, I. Javeri, K. Nellaiappan, A.M. Salazar, H. Daley, M. Seaman, E.I. Buchbinder, C.H. Yoon, M. Harden, N. Lennon, S. Gabriel, S.J. Rodig, D.H. Barouch, J.C. Aster, G. Getz, K. Wucherpfennig, D. Neuberg, J. Ritz, E.S. Lander, E.F. Fritsch, N. Hacohen, C.J. Wu, An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547(7662), 217–221 (2017)
M.C. Sellars, C.J. Wu, E.F. Fritsch, Cancer vaccines: Building a bridge over troubled waters. Cell 185(15), 2770–2788 (2022)
S.H. Chiou, D. Tseng, A. Reuben, V. Mallajosyula, I.S. Molina, S. Conley, J. Wilhelmy, A.M. McSween, X. Yang, D. Nishimiya, R. Sinha, B.Y. Nabet, C. Wang, J.B. Shrager, M.F. Berry, L. Backhus, N.S. Lui, H.A. Wakelee, J.W. Neal, S.K. Padda, G.J. Berry, A. Delaidelli, P.H. Sorensen, E. Sotillo, P. Tran, J.A. Benson, R. Richards, L. Labanieh, D.D. Klysz, D.M. Louis, S.A. Feldman, M. Diehn, I.L. Weissman, J. Zhang, I.I. Wistuba, P.A. Futreal, J.V. Heymach, K.C. Garcia, C.L. Mackall, M.M. Davis, Global analysis of shared T cell specificities in human non-small cell lung cancer enables HLA inference and antigen discovery. Immunity 54(3), 586-602.e8 (2021)
P.C. Rosato, S. Wijeyesinghe, J.M. Stolley, C.E. Nelson, R.L. Davis, L.S. Manlove, C.A. Pennell, B.R. Blazar, C.C. Chen, M.A. Geller, V. Vezys, D. Masopust, Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 10(1), 567 (2019)
R.S. Andersen, C.A. Thrue, N. Junker, R. Lyngaa, M. Donia, E. Ellebæk, I.M. Svane, T.N. Schumacher, P. Thor Straten, S.R. Hadrup, Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 72(7), 1642–1650 (2012)
Y. Simoni, E. Becht, M. Fehlings, C.Y. Loh, S.L. Koo, K.W.W. Teng, J.P.S. Yeong, R. Nahar, T. Zhang, H. Kared, K. Duan, N. Ang, M. Poidinger, Y.Y. Lee, A. Larbi, A.J. Khng, E. Tan, C. Fu, R. Mathew, M. Teo, W.T. Lim, C.K. Toh, B.H. Ong, T. Koh, A.M. Hillmer, A. Takano, T.K.H. Lim, E.H. Tan, W. Zhai, D.S.W. Tan, I.B. Tan, E.W. Newell, Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557(7706), 575–579 (2018)
I.K. Choi, Z. Wang, Q. Ke, M. Hong, D.W. Paul Jr., S.M. Fernandes, Z. Hu, J. Stevens, I. Guleria, H.J. Kim, H. Cantor, K.W. Wucherpfennig, J.R. Brown, J. Ritz, B. Zhang, Mechanism of EBV inducing anti-tumour immunity and its therapeutic use. Nature 590(7844), 157–162 (2021)
J.H. Newman, C.B. Chesson, N.L. Herzog, Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl. Acad. Sci. U S A 117(2), 1119–1128 (2020)
V. Gopalakrishnan, C.N. Spencer, L. Nezi, Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359(6371), 97–103 (2018)
C.A. Bessell, A. Isser, J.J. Havel, Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight. 5(8), e135597 (2020)
A. Fluckiger, R. Daillère, M. Sassi, Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369(6506), 936–942 (2020)
M. Röcken, J.F. Urban, E.M. Shevach, Infection breaks T-cell tolerance. Nature 359(6390), 79–82 (1992)
Funding
This investigation was supported by the Natural Science Foundation of China (81903020), the Natural Science Foundation of Hunan Province (2022JJ30072), the National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases (Lung Cancer).
Author information
Authors and Affiliations
Contributions
Ziyu Zhang: The main manuscript text writing, data curation, formal analysis, investigation, visualization, methodology, writing–original draft, writing–review and editing. Li Li: Fig. 4–5 preparation, data curation, formal analysis, investigation, visualization. Yang Gao: Resource, formal analysis, investigation. Xiaoxiong Xiao: Resource, formal analysis, investigation. Liyan Ji: Fig. 1–3 preparation, data curation, formal analysis, investigation, methodology. Zhipeng Zhou: Fig. 1–3 preparation, data curation, formal analysis, investigation, methodology. Juan Jiang: Resources, data curation, formal analysis, investigation. Shiqing Liu: Data curation, formal analysis, investigation. Jian An: Resources, data curation, formal analysis, investigation. Pengbo Deng: Resources, data curation, formal analysis, investigation. NanNan Du: Data curation, formal analysis, investigation. Pansong Li: Data curation, formal analysis, investigation. Xuefeng Xia: Investigation, methodology. Chengping Hu: Conceptualization, formal analysis, supervision, funding acquisition, methodology, writing–review, and editing. Min Li: Conceptualization, resources, formal analysis, supervision, funding acquisition, visualization, writing–original draft, project administration, writing–review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board (IRB) of Xiangya Hospital. The study was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Written informed content was obtained from every patient.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13402_2023_873_MOESM2_ESM.tif
Supplementary file2 Figure S1 Correlation and comparison of TCR diversity among tumor, LNs, and blood. (A) The association of Shannon and clonality between tumor and age using Pearson coefficiency. (B) The correlation of Shannon and clonality between blood and age using Pearson coefficiency. Comparison of clonality (C) and Shannon (D) at different tumor stages. (E) TCR diversity on different pathological tumors. (F) Pearson coefficiency of Shannon between tumor and blood at metastasis and non-metastatic patients. (G) The correlation of Shannon between LNs and tumor or PBMC (TIF 5500 KB)
13402_2023_873_MOESM3_ESM.tif
Supplementary file3 Figure S2 Comparison of Shannon and richness among LNs, tumor, and blood. The violin plots showing Shannon index (A) and richness (B) among blood, LNs and tumor. Two sided Mann-Whitney U test was performed to test the significance between groups. The box plots depicting Shannon index (C) and richness (D) of tumor, LNs, and blood between metastatic and non-metastatic patients (TIF 3000 KB)
13402_2023_873_MOESM4_ESM.jpg
Supplementary file4 Figure S3 T cell subsets and TCR diversity/characterization in LNs. (A) The Pearson coefficiency of clonality between tumor and LNs at the presence (left panel) or absence (middle panel) of metastasis in metastatic patients and non-metastatic patients (right panel). (B) Comparison of clonality among MPLN, MNLN, and NMLN at different lymph node staging. (C) The density of CD4+ and CD8+cells among MPLN, MNLN, and NMLN. (D) The ratio of viral-associated TCRs to non-viral TCRs among MPLN, MNLN, and NMLN. (E) The bar plot illustrates the length of CDR3 amino acid between metastasis-positive LNs and metastasis-negative LNs (JPG 1115 KB)
13402_2023_873_MOESM5_ESM.tif
Supplementary file5 Figure S4 Box plots depicting the frequency of V gene and J genes among MPLN, MNLN, and NMLN (n =134). Two-sided Man-Whitney U test (TIF 2733 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Li, L., Gao, Y. et al. Immune characteristics associated with lymph node metastasis in early-stage NSCLC. Cell Oncol. 47, 447–461 (2024). https://doi.org/10.1007/s13402-023-00873-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00873-y