Abstract
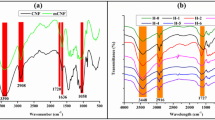
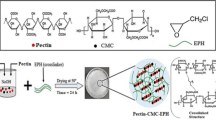
New cellulose-based hydrogels have been synthetically developed by a “one-step” procedure using cellulose, which was dissolved immediately in a NaOH/urea solution, and epichlorohydrin as a cross-linking agent. The ratios of cellulose and epichlorohydrin blend employed for the preparation of the hydrogel were 1:1, 1:2, 2:3, and 2:1. This polymerization process was investigated, and the obtained hydrogels were analyzed for their appearance, yield percentage, and water absorption capacity. Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), scanning electron microscope (SEM) analysis, and XRD were additionally explored in regard to the hydrogel samples. As the industry of textiles generates significant wastewater volumes that contain dangerous substances including dyes, in this study, we sought to utilize the hydrogel samples for the absorption of Safranin O which may be found in wastewater. The hydrogel showed an interesting ability to adsorb and remove Safranin O (SF) dye of up to 224 mg/g. Thermodynamically, the biosorption process was found to be exothermic (∆H < 0) and spontaneous (∆G < 0). The equilibrium results for SF removal by the biosorbent fits the Langmuir model well, indicating that the biosorption of SF which occurs in a monolayer was manifested primarily through the electrostatic driving force pathway. Response surface methodology calculations showed a maximum SF biosorption efficiency of 93.15% under optimal biosorption conditions: pH 6, biosorbent dose of 0.1 g, initial SF concentration of 10 mg L−1, and 90 min adsorption time at 25°C. Regeneration data further indicated that G2 could effectively be reused for up to three cycles, losing only 10% of the initial SF removal efficiency. Finally, it was concluded that the hydrogel (G2) could be used as a cost-effective, environmentally friendly, and recyclable biosorbent to remove SF dye from water.
















Similar content being viewed by others
Data availability
No specific datasets or materials were used in this article.
References
Tan B, Ching Y, Poh S et al (2015) A review of natural fiber reinforced poly(vinyl alcohol) based composites: application and opportunity. Polymers 7:2205–2222. https://doi.org/10.3390/polym7111509
Kadry G, Aboelmagd EI, Ibrahim MM (2019) Cellulosic-based hydrogel from biomass material for removal of metals from waste water. J Macromol Sci Part A 56:968–981. https://doi.org/10.1080/10601325.2019.1640063
Tamaddon F, Arab D, Ahmadi-AhmadAbadi E (2020) Urease immobilization on magnetic micro/nano-cellulose dialdehydes: urease inhibitory of Biginelli product in Hantzsch reaction by urea. Carbohydr Polym 229:115471. https://doi.org/10.1016/j.carbpol.2019.115471
Khenblouche A, Bechki D, Gouamid M et al (2019) Extraction and characterization of cellulose microfibers from Retama raetam stems. Polímeros 29:e2019011. https://doi.org/10.1590/0104-1428.05218
Oun AA, Shankar S, Rhim J-W (2020) Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit Rev Food Sci Nutr 60:435–460. https://doi.org/10.1080/10408398.2018.1536966
Imgharn A, Ighnih H, Hsini A et al (2021) Synthesis and characterization of polyaniline-based biocomposites and their application for effective removal of Orange G dye using adsorption in dynamic regime. Chem Phys Lett 778:138811. https://doi.org/10.1016/j.cplett.2021.138811
Ait El Fakir A, Anfar Z, Amedlous A et al (2021) Engineering of new hydrogel beads based conducting polymers: Metal-free catalysis for highly organic pollutants degradation. Appl Catal B Environ 286:119948. https://doi.org/10.1016/j.apcatb.2021.119948
Liu X, Zhou Z, Yin J et al (2020) Fast and environmental-friendly approach towards uniform hydrogel particles with ultrahigh and selective removal of anionic dyes. J Environ Chem Eng 8:104352. https://doi.org/10.1016/j.jece.2020.104352
El Messaoudi N, El Mouden A, Fernine Y et al (2022) Green synthesis of Ag2O nanoparticles using Punica granatum leaf extract for sulfamethoxazole antibiotic adsorption: characterization, experimental study, modeling, and DFT calculation. Environ Sci Pollut Res 30:81352–81369. https://doi.org/10.1007/s11356-022-21554-7
El Messaoudi N, Ciğeroğlu Z, Şenol ZM et al (2023) A comparative review of the adsorption and photocatalytic degradation of tetracycline in aquatic environment by g-C3N4-based materials. J Water Process Eng 55:104150. https://doi.org/10.1016/j.jwpe.2023.104150
Arslan DŞ, Ertap H, Şenol ZM et al (2023) Preparation of polyacrylamide titanium dioxide hybrid nanocomposite by direct polymerization and its applicability in removing crystal violet from aqueous solution. J Polym Environ. https://doi.org/10.1007/s10924-023-03004-8
Qin X, Lu A, Zhang L (2013) Gelation behavior of cellulose in NaOH/urea aqueous system via cross-linking. Cellulose 20:1669–1677. https://doi.org/10.1007/s10570-013-9961-z
Amri AE, Bensalah J, Essaadaoui Y et al (2022) Elaboration, characterization and performance evaluation of a new environmentally friendly adsorbent material based on the reed filter (Typha latifolia): kinetic and thermodynamic studies and application in the adsorption of Cd (II) ion. Chem Data Collect 39:100849. https://doi.org/10.1016/j.cdc.2022.100849
Elamri A, Kadiri L, Hsissou R et al (2022) Investigation of Typha latifolia (TL) as potential biosorbent for removal of the methyl orange anionic dye in the aqueous solution. Kinetic and DFT approaches. J Mol Struct 134098. https://doi.org/10.1016/j.molstruc.2022.134098
Bensalah J, Habsaoui A, Dagdag O et al (2021) Adsorption of a cationic dye (Safranin) by artificial cationic resins Amberlite®IRC-50: equilibrium, kinetic and thermodynamic study. Chem Data Collect 35:100756. https://doi.org/10.1016/j.cdc.2021.100756
Lebkiri I, Abbou B, Kadiri L et al (2022) Polyacrylamide hydrogel an effective adsorbent for the removal of heavy metal from aqueous solution: isotherm, kinetic, and thermodynamic studies. Russ J Phys Chem A 96:1484–1492. https://doi.org/10.1134/S0036024422070159
Ba Mohammed B, Hsini A, Abdellaoui Y et al (2020) Fe-ZSM-5 zeolite for efficient removal of basic Fuchsin dye from aqueous solutions: synthesis, characterization and adsorption process optimization using BBD-RSM modeling. J Environ Chem Eng 8:104419. https://doi.org/10.1016/j.jece.2020.104419
Amigun AT, Adekola FA, Tijani JO, Mustapha S (2022) Photocatalytic degradation of malachite green dye using nitrogen/sodium/iron-TiO2 nanocatalysts. Results Chem 4:100480. https://doi.org/10.1016/j.rechem.2022.100480
Ouass A, Kadiri L, Essaadaoui Y et al (2018) Removal of trivalent chromium ions from aqueous solutions by sodium polyacrylate beads. Mediterr J Chem 7:125–134. https://doi.org/10.13171/mjc72/01808051520-ouass
Abbou B, Lebki̇Ri̇ İ, Ouaddari H et al (2021) Kinetic and thermodynamic study on adsorption of cadmium from aqueous solutions using natural clay. J Turk Chem Soc Sect Chem 677–692. https://doi.org/10.18596/jotcsa.882016
Bensalah J, Amri AE, Ouass A et al (2022) Investigation of the cationic resin Am®IRC-50 as a potential adsorbent of Co (II): equilibrium isotherms and thermodynamic studies. Chem Data Collect 39:100879. https://doi.org/10.1016/j.cdc.2022.100879
Elabboudi M, Bensalah J, Amri AE et al (2023) Adsorption performance and mechanism of anionic MO dye by the adsorbent polymeric Amberlite®IRA-410 resin from environment wastewater: equilibrium kinetic and thermodynamic studies. J Mol Struct 1277:134789. https://doi.org/10.1016/j.molstruc.2022.134789
Kadiri L, Ouass A, Hsissou R et al (2021) Adsorption properties of coriander seeds: spectroscopic kinetic thermodynamic and computational approaches. J Mol Liq 343:116971. https://doi.org/10.1016/j.molliq.2021.116971
El Amri A, Ouass A, bensalah jaouad et al (2022) Extraction and characterization of cellulosic nanocrystals from stems of the reed plant large-leaved cattail (Typha latifolia). Mater Today Proc S2214785322056000. https://doi.org/10.1016/j.matpr.2022.08.408
Laabd M, Brahmi Y, El Ibrahimi B et al (2021) A novel mesoporous Hydroxyapatite@Montmorillonite hybrid composite for high-performance removal of emerging Ciprofloxacin antibiotic from water: Integrated experimental and Monte Carlo computational assessment. J Mol Liq 338:116705. https://doi.org/10.1016/j.molliq.2021.116705
Hsini A, Naciri Y, Benafqir M et al (2021) Facile synthesis and characterization of a novel 1,2,4,5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water. J Colloid Interface Sci 585:560–573. https://doi.org/10.1016/j.jcis.2020.10.036
Ait Ahsaine H, Zbair M, Anfar Z et al (2018) Cationic dyes adsorption onto high surface area “almond shell” activated carbon: kinetics, equilibrium isotherms and surface statistical modeling. Mater Today Chem 8:121–132. https://doi.org/10.1016/j.mtchem.2018.03.004
Laabd M, Imgharn A, Hsini A et al (2022) Efficient detoxification of Cr(VI)-containing effluents by sequential adsorption and reduction using a novel cysteine-doped PANi@faujasite composite: experimental study supported by advanced statistical physics prediction. J Hazard Mater 422:126857. https://doi.org/10.1016/j.jhazmat.2021.126857
Rahman ML, Biswas TK, Sarkar SM et al (2017) Adsorption of rare earth metals from water using a kenaf cellulose-based poly(hydroxamic acid) ligand. J Mol Liq 243:616–623. https://doi.org/10.1016/j.molliq.2017.08.096
Zalaghi A, Lamchouri F, Toufik H, Merzouki M (2014) Valorisation des matériaux naturels poreux dans le traitement des Lixiviats de la décharge publique non contrôlée de la ville de Taza (Valorization of natural porous materials in the treatment of leachate from the landfill uncontrolled city of Taza). 10
Zheng L, Meng P (2016) Preparation, characterization of corn stalk xanthates and its feasibility for Cd (II) removal from aqueous solution. J Taiwan Inst Chem Eng 58:391–400. https://doi.org/10.1016/j.jtice.2015.06.017
Pandey VC, Singh N, Singh RP, Singh DP (2014) Rhizoremediation potential of spontaneously grown Typha latifolia on fly ash basins: study from the field. Ecol Eng 71:722–727. https://doi.org/10.1016/j.ecoleng.2014.08.002
Ji F, Li C, Tang B et al (2012) Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution. Chem Eng J 209:325–333. https://doi.org/10.1016/j.cej.2012.08.014
Abdulhameed AS, Firdaus Hum NNM, Rangabhashiyam S et al (2021) Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. J Environ Chem Eng 9:105530. https://doi.org/10.1016/j.jece.2021.105530
Essekri A, Aarab N, Hsini A et al (2022) Enhanced adsorptive removal of crystal violet dye from aqueous media using citric acid modified red-seaweed: experimental study combined with RSM process optimization. J Dispers Sci Technol 43:1359–1372. https://doi.org/10.1080/01932691.2020.1857263
Hashem MA, Elnagar MM, Kenawy IM, Ismail MA (2020) Synthesis and application of hydrazono-imidazoline modified cellulose for selective separation of precious metals from geological samples. Carbohydr Polym 237:116177. https://doi.org/10.1016/j.carbpol.2020.116177
Vishan I, Saha B, Sivaprakasam S, Kalamdhad A (2019) Evaluation of Cd(II) biosorption in aqueous solution by using lyophilized biomass of novel bacterial strain Bacillus badius AK: biosorption kinetics, thermodynamics and mechanism. Environ Technol Innov 14:100323. https://doi.org/10.1016/j.eti.2019.100323
Bondock S, El-Zahhar AA, Alghamdi MM, Keshk SMAS (2019) Synthesis and evaluation of N-allylthiourea-modified chitosan for adsorptive removal of arsenazo III dye from aqueous solutions. Int J Biol Macromol 137:107–118. https://doi.org/10.1016/j.ijbiomac.2019.06.193
Franco DSP, Cunha JM, Dortzbacher GF, Dotto GL (2017) Adsorption of Co(II) from aqueous solutions onto rice husk modified by ultrasound assisted and supercritical technologies. Process Saf Environ Prot 109:55–62. https://doi.org/10.1016/j.psep.2017.03.029
Bediako JK, Wei W, Yun Y-S (2016) Conversion of waste textile cellulose fibers into heavy metal adsorbents. J Ind Eng Chem 43:61–68. https://doi.org/10.1016/j.jiec.2016.07.048
Ciğeroğlu Z, Kazan-Kaya ES, El Messaoudi N et al (2023) Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: Optimization, modeling, and theoretical calculation. J Mol Liq 369:120866. https://doi.org/10.1016/j.molliq.2022.120866
Hsini A, Naciri Y, Laabd M et al (2020) Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: equilibrium, reusability and process optimization. J Mol Liq 316:113832. https://doi.org/10.1016/j.molliq.2020.113832
Şenol ZM, Messaoudi NE, Fernine Y, Keskin ZS (2023) Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): experimental and DFT modeling studies. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-03781-1
Mohammadzadeh Pakdel P, Peighambardoust SJ, Arsalani N, Aghdasinia H (2022) Safranin-O cationic dye removal from wastewater using carboxymethyl cellulose-grafted-poly(acrylic acid-co-itaconic acid) nanocomposite hydrogel. Environ Res 212:113201. https://doi.org/10.1016/j.envres.2022.113201
Çetinkaya S, Kaya S, Aksu A et al (2023) Equilibrium and DFT modeling studies for the biosorption of Safranin O dye from water samples using Bacillus subtilis biosorbent. J Mol Struct 1276:134761. https://doi.org/10.1016/j.molstruc.2022.134761
Abukhadra MR, Adlii A, El-Sherbeeny AM et al (2020) Promoting the decontamination of different types of water pollutants (Cd2+, safranin dye, and phosphate) using a novel structure of exfoliated bentonite admixed with cellulose nanofiber. J Environ Manage 273:111130. https://doi.org/10.1016/j.jenvman.2020.111130
Ghosh I, Kar S, Chatterjee T et al (2021) Adsorptive removal of Safranin-O dye from aqueous medium using coconut coir and its acid-treated forms: adsorption study, scale-up design, MPR and GA-ANN modeling. Sustain Chem Pharm 19:100374. https://doi.org/10.1016/j.scp.2021.100374
Author information
Authors and Affiliations
Contributions
Azeddine Lebkiri: writing–original draft, data curation, conceptualization, visualization; Abdelhay El Amri: conceptualization, methodology, supervision, resources, funding acquisition, data curation, writing–review and editing; Assia Jebli: visualization, writing–review and editing; Basma Zarrik: conceptualization, writing–review and editing; Khadija Mortadi: data curation, formal analysis, conceptualization; Otmane Mqadmi: conceptualization, writing–review and editing; Rachid Hsissou: review and editing; El mahdi Hbaiz: conceptualization, validation, visualization; El Housseine Rifi: conceptualization, validation, visualization; Ahmed Lebkiri: conceptualization, validation, visualization.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lebkiri, A., Amri, A.E., Jebli, A. et al. Experimental study combined with RSM process optimization for removal of the (Safranin O) cationic dye in the aqueous solution using a hydrogel prepared based on cellulosic biomass: an effective and ecological approach. Biomass Conv. Bioref. 14, 9867–9886 (2024). https://doi.org/10.1007/s13399-024-05398-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-024-05398-4