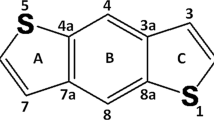
Abstract
The current research was aimed to examine the NLO properties of novel benzodithiophene-based donor–acceptor (D-A)-type compounds (BDTD1–BDTD8) via structural modulation of reference compound (BDTR). Optimization of the reference compound (BDTR) along with eight derivatives was accomplished at M06/6-311G(d,p) level. Subsequently, the optimized geometries were further employed to execute additional analyses: UV–Vis absorption, natural population analysis (NPA), NLO, frontier molecular orbitals (FMOs) and natural bond orbital (NBO) properties at the above-mentioned functional. All the tailored compounds (BDTD1–BDTD8) were reported with less energy difference in comparison with BDTR. The descending order of compounds accordant to Egap values was found to be as BDTR > BDTD1 > BDTD8 > BDTD4 > BDTD2 > BDTD5 > BDTD7 > BDTD6 > BDTD3. Furthermore, assisted by FMOs analysis, density of states (DOS) computations demonstrated significant charge mobility from HOMO towards LUMO in the derivatives. Global reactivity descriptors correspond to Egap values, BDTD3 with least energy difference accompanied with less hardness (1.028 eV) and highest softness (0.486 eV) among all the derivatives. BDTD3 exhibited the highest value of λmax (717.211 nm) in all the designed compounds relative to BDTR (576.161 nm). For all fabricated chromophores, the βtot values are presented in the decreasing order: BDTD3 > BDTD7 > BDTD6 > BDTD5 > BDTD2 > BDTD4 > BDTD1 > BDTD8 > BDTR. Interestingly, the highest values as 2.324 × 10–27 and 4.302 × 10–32 esu of βtot and γtot, respectively, were indicated by BDTD3 compound. Valuable NLO findings explored that our derivatives might be significant candidates for nonlinear optics.









Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Sivasankari, B.; Roopan, S.M.: L-Malic acid-doped Guanidinium Carbonate crystal: a New NLO material and its photoluminescence study. Optik 226, 165909 (2021)
Xu, H.-L.; Sun, S.-L.; Muhammad, S.; Su, Z.-M.: Three-propeller-blade-shaped electride: remarkable alkali-metal-doped effect on the first hyperpolarizability. Theoret. Chem. Acc. 128, 241–248 (2011)
Khalid, M.; Ali, A.; Jawaria, R.; Asghar, M.A.; Asim, S.; Khan, M.U.; Hussain, R.; Ur Rehman, M.F.; Ennis, C.J.; Akram, M.S.: First principles study of electronic and nonlinear optical properties of A-D–π–A and D–A–D–π–A configured compounds containing novel quinoline–carbazole derivatives. RSC Advances. 10, 22273–22283 (2020)
Saeed, A.; Muhammad, S.; Rehman, S.; Bibi, S.; Al-Sehemi, A.G.; Khalid, M.: Exploring the impact of central core modifications among several push-pull configurations to enhance nonlinear optical response. J. Mol. Graph. Model. 100, 107665 (2020)
Khan, M.U.; Khalid, M.; Ibrahim, M.; Braga, A.A.C.; Safdar, M.; Al-Saadi, A.A.; Janjua, M.R.S.A.: First theoretical framework of triphenylamine–dicyanovinylene-based nonlinear optical dyes: structural modification of π-linkers. J. Phys. Chem. C. 122, 4009–4018 (2018)
Kosar, N.; Ayub, K.; Mahmood, T.: Surface functionalization of twisted graphene C32H15 and C104H52 derivatives with alkalis and superalkalis for NLO response; a DFT study. J. Mol. Graph. Model. 102, 107794 (2021)
Zhong, R.-L.; Xu, H.-L.; Muhammad, S.; Zhang, J.; Su, Z.-M.: The stability and nonlinear optical properties: encapsulation of an excess electron compound LiCN⋯ Li within boron nitride nanotubes. J. Mater. Chem. 22, 2196–2202 (2012)
Vasumathi, S.; Jeyakumar, H.J.; Selvarajan, P.: Spectral, NLO, thermal, hardness and SEM studies of phosphate doped bis-urea oxalic acid crystals for laser applications. Chin. J. Phys. 73, 1–12 (2021)
Ahsin, A.; Ayub, K.: Remarkable electronic and NLO properties of bimetallic superalkali clusters: a DFT study. J. Nanostruct. Chem. 2, 1–17 (2021)
Khan, B.; Khalid, M.; Shah, M.R.; Tahir, M.N.; Asif, H.M.; Aliabad, H.A.R.; Hussain, A.: Synthetic, spectroscopic, SC-XRD and nonlinear optical analysis of potent hydrazide derivatives: a comparative experimental and DFT/TD-DFT exploration. J. Mol. Struct. 1200, 127140 (2020)
Akram, M.; Adeel, M.; Khalid, M.; Tahir, M.N.; Khan, M.U.; Asghar, M.A.; Ullah, M.A.; Iqbal, M.: A combined experimental and computational study of 3-bromo-5-(2, 5-difluorophenyl) pyridine and 3, 5-bis (naphthalen-1-yl) pyridine: Insight into the synthesis, spectroscopic, single crystal XRD, electronic, nonlinear optical and biological properties. J. Mol. Struct. 1160, 129–141 (2018)
Ivanov, I.P.; Li, X.; Dolan, P.R.; Gu, M.: Nonlinear absorption properties of the charge states of nitrogen-vacancy centers in nanodiamonds. Opt. Lett. 38, 1358–1360 (2013)
Zhang, B.; Shi, G.; Yang, Z.; Zhang, F.; Pan, S.: Fluorooxoborates: beryllium-free deep-ultraviolet nonlinear optical materials without layered growth. Angew. Chem. Int. Ed. 56, 3916–3919 (2017)
Li, M.; Li, Y.; Zhang, H.; Wang, S.; Ao, Y.; Cui, Z.: Molecular engineering of organic chromophores and polymers for enhanced bulk second-order optical nonlinearity. J. Mater. Chem. C. 5, 4111–4122 (2017)
Zhao, Y.; Li, H.; Shao, Z.; Xu, W.; Meng, X.; Song, Y.; Hou, H.: Investigation of regulating third-order nonlinear optical property by coordination interaction. Inorg. Chem. 58, 4792–4801 (2019)
Issa, Y.M.; Abdel-Latif, S.A.; El-Ansary, A.L.; Hassib, H.B.: The synthesis, spectroscopic characterization, DFT/TD-DFT/PCM calculations of the molecular structure and NBO of the novel charge-transfer complexes of pyrazine Schiff base derivatives with aromatic nitro compounds. New J. Chem. 45, 1482–1499 (2021)
Abdel-Kader, N.S.; Abdel-Latif, S.A.; El-Ansary, A.L.; Sayed, A.G.: Spectroscopic studies, density functional theory calculations, non-linear optical properties, biological activity of 1-hydroxy-4-((4-(N-(pyrimidin-2-yl) sulfamoyl) phenyl) diazenyl)-2-naphthoic acid and its chelates with Nickel (II), Copper (II), Zinc (II) and Palladium (II) metal ions. J. Mol. Struct. 1223, 129203 (2021)
Janjua, M.R.S.A.: Nonlinear optical response of a series of small molecules: quantum modification of π-spacer and acceptor. J. Iran. Chem. Soc. 14, 2041–2054 (2017)
Coe, B.J.; Foxon, S.P.; Harper, E.C.; Raftery, J.; Shaw, R.; Swanson, C.A.; Asselberghs, I.; Clays, K.; Brunschwig, B.S.; Fitch, A.G.: Nonlinear optical and related properties of iron (II) pentacyanide complexes with quaternary nitrogen electron acceptor units. Inorg. Chem. 48, 1370–1379 (2009)
Janjua, M.R.S.A.; Jamil, S.; Mahmood, A.; Zafar, A.; Haroon, M.; Bhatti, H.N.: Solvent-dependent non-linear optical properties of 5, 5′-disubstituted-2, 2′-bipyridine complexes of ruthenium (II): a quantum chemical perspective. Aust. J. Chem. 68, 1502–1507 (2015)
Janjua, M.R.S.A.; Yamani, Z.H.; Jamil, S.; Mahmood, A.; Ahmad, I.; Haroon, M.; Tahir, M.H.; Yang, Z.; Pan, S.: First principle study of electronic and non-linear optical (NLO) properties of triphenylamine dyes: interactive design computation of new NLO compounds. Aust. J. Chem. 69, 467–472 (2015)
Lacroix, P.G.; Malfant, I.; Lepetit, C.: Second-order nonlinear optics in coordination chemistry: an open door towards multi-functional materials and molecular switches. Coord. Chem. Rev. 308, 381–394 (2016)
Kato, S.; Diederich, F.: Non-planar push–pull chromophores. Chem. Commun. 46, 1994–2006 (2010)
Roncali, J.: Molecular bulk heterojunctions: an emerging approach to organic solar cells. Acc. Chem. Res. 42, 1719–1730 (2009)
Mahmood, A.; Abdullah, M.I.; Nazar, M.F.: Quantum chemical designing of novel organic non-linear optical compounds. Bull. Korean Chem. Soc. 35, 1391–1396 (2014)
Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I.: Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 48, 1596–1625 (2019)
Liu, W.; Xu, X.; Yuan, J.; Leclerc, M.; Zou, Y.; Li, Y.: Low-bandgap non-fullerene acceptors enabling high-performance organic solar cells. ACS Energy Lett. 6, 598–608 (2021)
Lin, Y.; Zhan, X.: Non-fullerene acceptors for organic photovoltaics: an emerging horizon. Mater. Horiz. 1, 470–488 (2014)
Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J.: Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 139, 7148–7151 (2017)
Liang, N.; Jiang, W.; Hou, J.; Wang, Z.: New developments in non-fullerene small molecule acceptors for polymer solar cells. Mater. Chem. Front. 1, 1291–1303 (2017)
Saeed, M.U.; Iqbal, J.; Mehmood, R.F.; Akram, S.J.; El-Badry, Y.A.; Noor, S.; Khera, R.A.: End-capped modification of Y-Shaped dithienothiophen[3,2-b]-pyrrolobenzothiadiazole (TPBT) based non-fullerene acceptors for high performance organic solar cells by using DFT approach. Surf. Interfaces. 30, 101875 (2022)
Wang, L.; An, Q.; Yan, L.; Bai, H.-R.; Jiang, M.; Mahmood, A.; Yang, C.; Zhi, H.; Wang, J.-L.: Non-fullerene acceptors with hetero-dihalogenated terminals induce significant difference in single crystallography and enable binary organic solar cells with 17.5% efficiency. Energy Environ. Sci. 15, 320–333 (2022)
Ge, G.-Y.; Li, J.-T.; Wang, J.-R.; Xiong, M.; Dong, X.; Li, Z.-J.; Li, J.-L.; Cao, X.-Y.; Lei, T.; Wang, J.-L.: Unveiling the interplay among end group, molecular packing, doping level, and charge transport in N-doped small-molecule organic semiconductors. Adv. Func. Mater. 32, 2108289 (2022)
Yao, C.; Yang, Y.; Li, L.; Bo, M.; Peng, C.; Huang, Z.; Wang, J.: Replacing the cyano (–C [triple bond, length as m-dash] N) group to design environmentally friendly fused-ring electron acceptors. Phys. Chem. Chem. Phys. 23, 18085–18092 (2021)
Yu, Q.; Xu, J.; Fu, J.; Xu, T.; Yan, X.; Chen, S.; Chen, H.; Sun, K.; Kan, Z.; Lu, S.: Crystallinity dictates the selection of fullerene or non-fullerene acceptors in a small molecule organic solar cell. Dyes Pigm. 187, 109085 (2021)
Frisch, M.J.; Clemente, F.R.: Gaussian 09, revision a. 01, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, V. Barone, B. Mennucci, GA Petersson, H. Nakatsuji, M. Caricato, X. Li, HP Hratchian, AF Izmaylov, J. Bloino, G. Zhe. 20–44 (2009)
Bryantsev, V.S.; Diallo, M.S.; Van Duin, A.C.; Goddard, W.A., III.: Evaluation of B3LYP, X3LYP, and M06-class density functionals for predicting the binding energies of neutral, protonated, and deprotonated water clusters. J. Chem. Theory Comput. 5, 1016–1026 (2009)
Bibi, A.; Muhammad, S.; UrRehman, S.; Bibi, S.; Bashir, S.; Ayub, K.; Adnan, M.; Khalid, M.: Chemically modified quinoidal oligothiophenes for enhanced linear and third-order nonlinear optical properties. ACS Omega 6, 24602 (2021)
Khalid, M.; Khan, M.U.; Hussain, R.; Irshad, S.; Ali, B.; Braga, A.A.C.; Imran, M.; Hussain, A.: Exploration of second and third order nonlinear optical properties for theoretical framework of organic D–π–D–π–A type compounds. Opt. Quant. Electron. 53, 1–19 (2021)
Hudson, J.J.; Sauer, B.E.; Tarbutt, M.R.; Hinds, E.A.: Measurement of the electron electric dipole moment using YbF molecules. Phys. Rev. Lett. 89, 023003 (2002)
Alparone, A.: Linear and nonlinear optical properties of nucleic acid bases. Chem. Phys. 410, 90–98 (2013)
Oudar, J.-L.; Chemla, D.S.: Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 66, 2664–2668 (1977)
Naik, V.S.; Patil, P.S.; Wong, Q.A.; Quah, C.K.; Gummagol, N.B.; Jayanna, H.S.: Molecular structure, linear optical, second and third-order nonlinear optical properties of two non-centrosymmetric thiophene-chalcone derivatives. J. Mol. Struct. 1222, 128901 (2020)
Dennington, R.; Keith, T.; Millam, J.: Semichem Inc. Shawnee Mission KS, GaussView, Version. 5, (2009)
Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R.: Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 1–17 (2012)
Zhurko, G.A.: Chemcraft: http://www.chemcraftprog. com. Received: October. 22, (2014)
O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M.: Cclib: a library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839–845 (2008)
Lu, T.; Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012)
Afzal, Z.; Hussain, R.; Khan, M.U.; Khalid, M.; Iqbal, J.; Alvi, M.U.; Adnan, M.; Ahmed, M.; Mehboob, M.Y.; Hussain, M.: Designing indenothiophene-based acceptor materials with efficient photovoltaic parameters for fullerene-free organic solar cells. J. Mol. Model. 26, 1–17 (2020)
Hussain, R.; Khan, M.U.; Mehboob, M.Y.; Khalid, M.; Iqbal, J.; Ayub, K.; Adnan, M.; Ahmed, M.; Atiq, K.; Mahmood, K.: Enhancement in photovoltaic properties of N, N-diethylaniline based donor materials by bridging core modifications for efficient solar cells. ChemistrySelect 5, 5022–5034 (2020)
Khan, M.U.; Hussain, R.; Mehboob, M.Y.; Khalid, M.; Ehsan, M.A.; Rehman, A.; Janjua, M.R.S.A.: First theoretical framework of Z-shaped acceptor materials with fused-chrysene core for high performance organic solar cells. Spectrochimica Acta Part A. 245, 118938 (2021)
Haroon, M.; Al-Saadi, A.A.; Janjua, M.R.S.A.: Insights into end-capped modifications effect on the photovoltaic and optoelectronic properties of S-shaped fullerene-free acceptor molecules: a density functional theory computational study for organic solar cells. J. Phys. Org. Chem. 35, e4314 (2022)
Janjua, M.R.S.A.: Prediction and understanding: quantum chemical framework of transition metals enclosed in a B12N12 inorganic nanocluster for adsorption and removal of DDT from the environment. Inorg. Chem. 60, 10837–10847 (2021)
Janjua, M.R.S.A.: How does bridging core modification alter the photovoltaic characteristics of triphenylamine-based hole transport materials? Theoretical understanding and prediction. Chem. A Eur. J. 27, 4197–4210 (2021)
Adeel, M.; Khalid, M.; Ullah, M.A.; Muhammad, S.; Khan, M.U.; Tahir, M.N.; Khan, I.; Asghar, M.; Mughal, K.S. (2010) Exploration of CH⋯F & CF⋯H mediated supramolecular arrangements into fluorinated terphenyls and theoretical prediction of their third-order nonlinear optical response. RSC Adv. 11, 7766–7778.
Khalid, M.; Ali, A.; Abid, S.; Tahir, M.N.; Khan, M.U.; Ashfaq, M.; Imran, M.; Ahmad, A.: Facile ultrasound-based synthesis, SC-XRD, DFT exploration of the substituted acyl-hydrazones: an experimental and theoretical slant towards supramolecular chemistry. ChemistrySelect 5, 14844–14856 (2020)
Jiao, C.; Guo, Z.; Sun, B.; Meng, L.; Wan, X.; Zhang, M.; Zhang, H.; Li, C.; Chen, Y.: An acceptor–donor–acceptor type non-fullerene acceptor with an asymmetric backbone for high performance organic solar cells. J. Mater. Chem. C. 8, 6293–6298 (2020)
Cai, K.; Wu, H.; Hua, T.; Liao, C.; Tang, H.; Wang, L.; Cao, D.: Molecular engineering of the fused azacycle donors in the D-A-π-A metal-free organic dyes for efficient dye-sensitized solar cells. Dyes Pigm. 197, 109922 (2022)
Haroon, M.; Janjua, M.R.S.A.: Prediction of NLO response of substituted organoimido hexamolybedate: First theoretical framework based on p-anisidine adduct [Mo6O18 (p-MeOC6H4N)] 2. Mater. Today Commun. 26, 101880 (2021)
Jezuita, A.; Ejsmont, K.; Szatylowicz, H.: Substituent effects of nitro group in cyclic compounds. Struct. Chem. 32, 179–203 (2021)
Pham, P.-T.; Xia, Y.; Frisbie, C.D.; Bader, M.M.: Single crystal field effect transistor of a Y-shaped ladder-type oligomer. J. Phys. Chem. C. 112, 7968–7971 (2008)
Cai, X.; Burand, M.W.; Newman, C.R.; da Silva Filho, D.A.; Pappenfus, T.M.; Bader, M.M.; Brédas, J.-L.; Mann, K.R.; Frisbie, C.D.: N-and P-channel transport behavior in thin film transistors based on tricyanovinyl-capped oligothiophenes. J. Phys. Chem. B 110, 14590–14597 (2006)
Seo, D.-K.; Hoffmann, R.: Direct and indirect band gap types in one-dimensional conjugated or stacked organic materials. Theoret. Chem. Acc. 102, 23–32 (1999)
Pearson, R.G.: Absolute electronegativity and absolute hardness of Lewis acids and bases. J. Am. Chem. Soc. 107, 6801–6806 (1985)
Parr, R.G.; Yang, W.: Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 106, 4049–4050 (1984)
Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E.: Electronegativity: the density functional viewpoint. J. Chem. Phys. 68, 3801–3807 (1978)
Parthasarathi, R.; Padmanabhan, J.; Elango, M.; Subramanian, V.; Chattaraj, P.K.: Intermolecular reactivity through the generalized philicity concept. Chem. Phys. Lett. 394, 225–230 (2004)
Parr, R.G.; Pearson, R.G.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983)
Politzer, P.; Truhlar, D.G.: Introduction: the role of the electrostatic potential in chemistry. Chem. Appl. 5, 1–6 (1981)
Chattaraj, P.K.; Roy, D.R.: Update 1 of: electrophilicity index. Chem. Rev. 107, PR46–PR74 (2007)
Amiri, S.S.; Makarem, S.; Ahmar, H.; Ashenagar, S.: Theoretical studies and spectroscopic characterization of novel 4-methyl-5-((5-phenyl-1, 3, 4-oxadiazol-2-yl) thio) benzene-1, 2-diol. J. Mol. Struct. 1119, 18–24 (2016)
Padmanabhan, J.; Parthasarathi, R.; Subramanian, V.; Chattaraj, P.K.: Electrophilicity-based charge transfer descriptor. J. Phys. Chem. A 111, 1358–1361 (2007)
Koopmans, T.: The classification of wave functions and eigen-values to the single electrons of an atom. Physica. 1, 104–113 (1934)
Mirkamali, E.S.; Ahmadi, R.; Kalateh, K.; Zarei, G.: 251. Adsorption of Melphalan anticancer drug on the surface of carbon nanotube: a comprehensive DFT study. Int. J. 9, 11 (2020)
Arshad, M.N.; Khalid, M.; Asad, M.; Braga, A.A.; Asiri, A.M.; Alotaibi, M.M.: Influence of peripheral modification of electron acceptors in nonfullerene (O-IDTBR1)-based derivatives on nonlinear optical response: DFT/TDDFT study. ACS Omega 7, 11631–11642 (2022)
Azeem, U.; Khera, R.A.; Naveed, A.; Imran, M.; Assiri, M.A.; Khalid, M.; Iqbal, J.: Tuning of a A-A–D–A–A-type small molecule with benzodithiophene as a central core with efficient photovoltaic properties for organic solar cells. ACS Omega 6, 28923–28935 (2021)
Khan, M.U.; Mehboob, M.Y.; Hussain, R.; Afzal, Z.; Khalid, M.; Adnan, M.: Designing spirobifullerene core based three-dimensional cross shape acceptor materials with promising photovoltaic properties for high-efficiency organic solar cells. Int. J. Quantum Chem. 120, e26377 (2020)
Mahmood, A.; Abdullah, M.I.; Khan, S.U.-D.: Enhancement of nonlinear optical (NLO) properties of indigo through modification of auxiliary donor, donor and acceptor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 139, 425–430 (2015)
Lu, X.: DC-C.: Fractal geometry and architecture design: case study review. Chaotic Model. Simul. (CMSIM). 311, 322 (2012)
Khalid, M.; Lodhi, H.M.; Khan, M.U.; Imran, M.: Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: a DFT approach. RSC Adv. 11, 14237–14250 (2021)
Mahmood, A.; Khan, S.U.-D.; Rana, U.A.; Tahir, M.H.: Red shifting of absorption maxima of phenothiazine based dyes by incorporating electron-deficient thiadiazole derivatives as π-spacer. Arab. J. Chem. 12, 1447–1453 (2019)
Adeoye, M.D.; Adeogun, A.I.; Adewuyi, S.; Ahmed, S.A.; Odozi, N.W.; Obi-Egbeedi, N.O.: Effect of solvents on the electronic absorption spectra of 9, 14 dibenzo (a, c) phenazine and tribenzo (a, c, i) phenazine. Sci. Res. Essays. 4, 107–111 (2009)
Uzun, S.; Esen, Z.; Koç, E.; Usta, N.C.; Ceylan, M.: Experimental and density functional theory (MEP, FMO, NLO, Fukui functions) and antibacterial activity studies on 2-amino-4-(4-nitrophenyl)-5, 6-dihydrobenzo [h] quinoline-3-carbonitrile. J. Mol. Struct. 1178, 450–457 (2019)
Khalid, M.; Arshad, M.N.; Murtaza, S.; Shafiq, I.; Haroon, M.; Asiri, A.M.; de AlcântaraMorais, S.F.; Braga, A.A.: Enriching NLO efficacy via designing non-fullerene molecules with the modification of acceptor moieties into ICIF2F: an emerging theoretical approach. RSC Adv. 12, 13412–13427 (2022)
Khalid, M.; Khan, M.U.; Shafiq, I.; Hussain, R.; Ali, A.; Imran, M.; Braga, A.A.; Fayyaz ur Rehman, M.; Akram, M.S.: Structural modulation of π-conjugated linkers in D–π–A dyes based on triphenylamine dicyanovinylene framework to explore the NLO properties. R. Soc. Open Sci. 8, 210570 (2021)
Won, Y.S.; Yang, Y.S.; Kim, J.H.; Ryu, J.-H.; Kim, K.K.; Park, S.S.: Organic photosensitizers based on terthiophene with alkyl chain and double acceptors for application in dye-sensitized solar cells. Energy Fuels 24, 3676–3681 (2010)
Ans, M.; Iqbal, J.; Ayub, K.; Ali, E.; Eliasson, B.: Spirobifluorene based small molecules as an alternative to traditional fullerene acceptors for organic solar cells. Mater. Sci. Semicond. Process. 94, 97–106 (2019)
Ans, M.; Iqbal, J.; Ahmad, Z.; Muhammad, S.; Hussain, R.; Eliasson, B.; Ayub, K.: Designing three-dimensional (3D) non-fullerene small molecule acceptors with efficient photovoltaic parameters. ChemistrySelect 3, 12797–12804 (2018)
Liu, Z.; Lu, T.; Chen, Q.: An sp-hybridized all-carboatomic ring, cyclo [18] carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 165, 461–467 (2020)
Liu, Z.; Lu, T.: Optical properties of novel conjugated nanohoops: Revealing the effects of topology and size. J. Phys. Chem. C. 124, 7353–7360 (2020)
Yuan, J.; Yuan, Y.; Tian, X.; Wang, H.; Liu, Y.; Feng, R.: Photoswitchable boronic acid derived salicylidenehydrazone enabled by photochromic spirooxazine and fulgide moieties: multiple responses of optical absorption, fluorescence emission, and quadratic nonlinear optics. J. Phys. Chem. C. 123, 29838–29855 (2019)
Hassan, T.; Hussain, R.; Khan, M.U.; Habiba, U.; Irshad, Z.; Adnan, M.; Lim, J.: Development of non-fused acceptor materials with 3D-Interpenetrated structure for stable and efficient organic solar cells. Mater. Sci. Semicond. Process. 151, 107010 (2022)
Köse, M.E.: Evaluation of acceptor strength in thiophene coupled donor–acceptor chromophores for optimal design of organic photovoltaic materials. J. Phys. Chem. A 116, 12503–12509 (2012)
Kim, B.-G.; Zhen, C.-G.; Jeong, E.J.; Kieffer, J.; Kim, J.: Organic dye design tools for efficient photocurrent generation in dye-sensitized solar cells: exciton binding energy and electron acceptors. Adv. Func. Mater. 22, 1606–1612 (2012)
Ans, M.; Iqbal, J.; Eliasson, B.; Ayub, K.: Opto-electronic properties of non-fullerene fused-undecacyclic electron acceptors for organic solar cells. Comput. Mater. Sci. 159, 150–159 (2019)
Shkir, M.; Muhammad, S.; AlFaify, S.; Chaudhry, A.R.; Al-Sehemi, A.G.: Shedding light on molecular structure, spectroscopic, nonlinear optical and dielectric properties of bis (thiourea) silver (I) nitrate single crystal: a dual approach. Arab. J. Chem. 12, 4612–4626 (2019)
Ali, A.; Khalid, M.; Rehman, M.F.U.; Haq, S.; Ali, A.; Tahir, M.N.; Ashfaq, M.; Rasool, F.; Braga, A.A.C.: Efficient synthesis, SC-XRD, and theoretical studies of O-Benzenesulfonylated pyrimidines: role of noncovalent interaction influence in their supramolecular network. ACS Omega 5, 15115–15128 (2020)
Yang, C.-C.; Zheng, X.-L.; Tian, W.Q.; Li, W.-Q.; Yang, L.: Tuning the edge states in X-type carbon based molecules for applications in nonlinear optics. Phys. Chem. Chem. Phys. 24, 7713–7722 (2022)
Khalid, M.; Khan, M.U.; Shafiq, I.; Hussain, R.; Mahmood, K.; Hussain, A.; Jawaria, R.; Hussain, A.; Imran, M.; Assiri, M.A.: NLO potential exploration for D–π–A heterocyclic organic compounds by incorporation of various π-linkers and acceptor units. Arab. J. Chem. 14, 103295 (2021)
Ashfaq, M.; Ali, A.; Tahir, M.N.; Khalid, M.; Assiri, M.A.; Imran, M.; Munawar, K.S.; Habiba, U.: Synthetic approach to achieve halo imine units: solid-state assembly, DFT based electronic and non linear optical behavior. Chem. Phys. Lett. 803, 139843 (2022)
Buvaneswari, M.; Santhakumari, R.; Usha, C.; Jayasree, R.; Sagadevan, S.: Synthesis, growth, structural, spectroscopic, optical, thermal, DFT, HOMO–LUMO, MEP, NBO analysis and thermodynamic properties of vanillin isonicotinic hydrazide single crystal. J. Mol. Struct. 1243, 130856 (2021)
Mahmood, A.; Khan, S.U.-D.; Rana, U.A.; Janjua, M.R.S.A.; Tahir, M.H.; Nazar, M.F.; Song, Y.: Effect of thiophene rings on UV/visible spectra and non-linear optical (NLO) properties of triphenylamine based dyes: a quantum chemical perspective. J. Phys. Org. Chem. 28, 418–422 (2015)
Adant, C.; Dupuis, M.; Bredas, J.L.: Ab initio study of the nonlinear optical properties of urea: electron correlation and dispersion effects. Int. J. Quantum Chem. 56, 497–507 (1995)
Demircioğlu, Z.; Kaştaş, G.; Kaştaş, Ç.A.; Frank, R.: Spectroscopic, XRD, Hirshfeld surface and DFT approach (chemical activity, ECT, NBO, FFA, NLO, MEP, NPA& MPA) of (E)-4-bromo-2-[(4-bromophenylimino) methyl]-6-ethoxyphenol. J. Mol. Struct. 1191, 129–137 (2019)
Demircioğlu, Z.; Kaştaş, Ç.A.; Büyükgüngör, O.: X-ray structural, spectroscopic and computational approach (NBO, MEP, NLO, NPA, fukui function analyses) of (E)-2-((4-bromophenylimino) methyl)-3-methoxyphenol. Mol. Cryst. Liq. Cryst. 656, 169–184 (2017)
Acknowledgements
Dr. Muhammad Khalid gratefully acknowledges the financial support of HEC Pakistan (project no. 20-14703/NRPU/R&D/HEC/2021). Authors are thankful for cooperation and collaboration of A.A.C.B from IQ-USP, Brazil, especially for his continuous support and providing computational laboratory facilities. A.A.C.B. (grant 2015/01491-3) is highly thankful to Fundação de Amparo à Pesquisa do Estado de São Paulo for the cooperation and financial assistance. The authors thank the Researchers Supporting Project number (RSP2023R29), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalid, M., Maqsood, R., Shafiq, I. et al. Theoretical Approach towards Benzodithiophene-Based Chromophores with Extended Acceptors for Prediction of Efficient Nonlinear Optical Behaviour. Arab J Sci Eng 49, 339–359 (2024). https://doi.org/10.1007/s13369-023-08136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08136-6