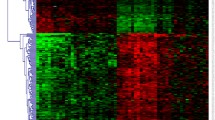
Abstract
Epigenetic changes such as DNA methylation were observed in drug-resistant temporal lobe epilepsy (DR-TLE), a disease that affects 25–30% of epilepsy patients. The main objective is to simultaneously describe DNA methylation patterns associated with DR-TLE in hippocampus, amygdala, surrounding cortex to the epileptogenic zone (SCEZ), and peripheral blood. An Illumina Infinium MethylationEPIC BeadChip array was performed in 19 DR-TLE patients and 10 postmortem non-epileptic controls. Overall, 32, 59, and 3210 differentially methylated probes (DMPs) were associated with DR-TLE in the hippocampus, amygdala, and SCEZ, respectively. These DMP-affected genes were involved in neurotrophic and calcium signaling in the hippocampus and voltage-gated channels in SCEZ, among others. One of the hippocampus DMPs (cg26834418 (CHORDC1)) showed a strong blood–brain correlation with BECon and IMAGE-CpG, suggesting that it could be a potential surrogate peripheral biomarker of DR-TLE. Moreover, in three of the top SCEZ’s DMPs (SHANK3, SBF1, and MCF2L), methylation status was verified with methylation-specific qPCR. The differentially methylated CpGs were classified in DMRs: 2 in the hippocampus, 12 in the amygdala, and 531 in the SCEZ. We identified genes that had not been associated to DR-TLE so far such as TBX5, EXOC7, and WRHN. The area with more DMPs associated with DR-TLE was the SCEZ, some of them related to voltage-gated channels. The DMPs found in the amygdala were involved in inflammatory processes. We also found a potential surrogate peripheral biomarker of DR-TLE. Thus, these results provide new insights into epigenetic modifications involved in DR-TLE.
Graphical Abstract





Similar content being viewed by others
Data Availability
All data produced in the present study are available upon reasonable request to the authors.
References
Amin U, Benbadis SR (2020) Avoiding complacency when treating uncontrolled seizures: why and how? Expert Rev Neurother 1–9.https://doi.org/10.1080/14737175.2020.1713100
Löscher W, Potschka H, Sisodiya SM, Vezzani A (2020) Drug Resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev 72:606–638. https://doi.org/10.1124/pr.120.019539
Fang M, Xi Z-Q, Wu Y, Wang X-F (2011) A new hypothesis of drug refractory epilepsy: neural network hypothesis. Med Hypotheses 76:871–876. https://doi.org/10.1016/j.mehy.2011.02.039
Torres CV, Pastor J, Garcia-Navarrete E et al (2015) Classification of structural lesions in magnetic resonance imaging. Surgical implications in drug-resistant epilepsy patients. Rev Neurol 61:241–248
Janmohamed M, Brodie MJ, Kwan P (2019) Pharmacoresistance - Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 107790. https://doi.org/10.1016/j.neuropharm.2019.107790
Kobow K, Reid CA, van Vliet EA et al (2020) Epigenetics explained: a topic “primer” for the epilepsy community by the ILAE Genetics/Epigenetics Task Force. Epileptic Disord Int Epilepsy J Videotape 22:127–141. https://doi.org/10.1684/epd.2020.1143
Conboy K, Henshall DC, Brennan GP (2021) Epigenetic principles underlying epileptogenesis and epilepsy syndromes. Neurobiol Dis 148:105179. https://doi.org/10.1016/j.nbd.2020.105179
Belhedi N, Perroud N, Karege F et al (2014) Increased CPA6 promoter methylation in focal epilepsy and in febrile seizures. Epilepsy Res 108:144–148. https://doi.org/10.1016/j.eplepsyres.2013.10.007
Kobow K, Ziemann M, Kaipananickal H et al (2019) Genomic DNA methylation distinguishes subtypes of human focal cortical dysplasia. Epilepsia 60:1091–1103. https://doi.org/10.1111/epi.14934
Kobow K, Jeske I, Hildebrandt M et al (2009) Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol 68:356–364. https://doi.org/10.1097/NEN.0b013e31819ba737
Long H-Y, Feng L, Kang J et al (2017) Blood DNA methylation pattern is altered in mesial temporal lobe epilepsy. Sci Rep 7:43810. https://doi.org/10.1038/srep43810
Lv Y, Zheng X, Shi M et al (2019) Different EPHX1 methylation levels in promoter area between carbamazepine-resistant epilepsy group and carbamazepine-sensitive epilepsy group in Chinese population. BMC Neurol 19:114. https://doi.org/10.1186/s12883-019-1308-4
Martins-Ferreira R, Leal B, Chaves J et al (2022) Epilepsy progression is associated with cumulative DNA methylation changes in inflammatory genes. Prog Neurobiol 209:102207. https://doi.org/10.1016/j.pneurobio.2021.102207
Miller-Delaney SFC, Bryan K, Das S et al (2015) Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 138:616–631. https://doi.org/10.1093/brain/awu373
Suchkova IO, Borisova EV, Patkin EL (2020) Length polymorphism and methylation status of UPS29 Minisatellite of the ACAP3 gene as molecular biomarker of epilepsy. Sex Differences in Seizure Types and Symptoms. Int J Mol Sci 21. https://doi.org/10.3390/ijms21239206
Xiao W, Liu C, Zhong K et al (2020) CpG methylation signature defines human temporal lobe epilepsy and predicts drug-resistant. CNS Neurosci Ther. https://doi.org/10.1111/cns.13394
Xiao W, Cao Y, Long H et al (2018) Genome-wide DNA methylation patterns analysis of noncoding RNAs in temporal lobe epilepsy patients. Mol Neurobiol 55:793–803. https://doi.org/10.1007/s12035-016-0353-x
Zhu Q, Wang L, Zhang Y et al (2012) Increased expression of DNA methyltransferase 1 and 3a in human temporal lobe epilepsy. J Mol Neurosci MN 46:420–426. https://doi.org/10.1007/s12031-011-9602-7
Mohandas N, Loke YJ, Hopkins S et al (2019) Evidence for type-specific DNA methylation patterns in epilepsy: a discordant monozygotic twin approach. Epigenomics 11:951–968. https://doi.org/10.2217/epi-2018-0136
Zhang W, Wang H, Liu B et al (2021) Differential DNA methylation profiles in patients with temporal lobe epilepsy and hippocampal sclerosis ILAE Type I. J Mol Neurosci MN 71:1951–1966. https://doi.org/10.1007/s12031-020-01780-9
Wang L, Fu X, Peng X et al (2016) DNA methylation profiling reveals correlation of differential methylation patterns with gene expression in human epilepsy. J Mol Neurosci MN 59:68–77. https://doi.org/10.1007/s12031-016-0735-6
Liu X, Ou S, Xu T et al (2016) New differentially expressed genes and differential DNA methylation underlying refractory epilepsy. Oncotarget. https://doi.org/10.18632/oncotarget.13642
Younus I, Reddy DS (2017) Epigenetic interventions for epileptogenesis: A new frontier for curing epilepsy. Pharmacol Ther 177:108–122. https://doi.org/10.1016/j.pharmthera.2017.03.002
Engel JJ, Van Ness, Rasmussen (1993) Outcome withrespect to epileptic seizures. In: Surgigal treatmentof the epilepsies. pp 609–21
Bibikova M, Le J, Barnes B et al (2009) Genome-wide DNA methylation profiling using Infinium ® assay. Epigenomics 1:177–200. https://doi.org/10.2217/epi.09.14
Maksimovic J, Phipson B, Oshlack A (2016) A cross-package bioconductor workflow for analysing methylation array data. F1000Research 5:1281. https://doi.org/10.12688/f1000research.8839.3
Triche TJ, Weisenberger DJ, Van Den Berg D et al (2013) Low-level processing of illumina infinium DNA methylation BeadArrays. Nucleic Acids Res 41:e90. https://doi.org/10.1093/nar/gkt090
Teschendorff AE, Marabita F, Lechner M et al (2013) A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinforma Oxf Engl 29:189–196. https://doi.org/10.1093/bioinformatics/bts680
Tian Y, Morris TJ, Webster AP et al (2017) ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinforma Oxf Engl 33:3982–3984. https://doi.org/10.1093/bioinformatics/btx513
Chen Y, Lemire M, Choufani S et al (2013) Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics 8:203–209. https://doi.org/10.4161/epi.23470
Pidsley R, Zotenko E, Peters TJ et al (2016) Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol 17:208. https://doi.org/10.1186/s13059-016-1066-1
Ritchie ME, Phipson B, Wu D et al (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47–e47. https://doi.org/10.1093/nar/gkv007
Kuleshov MV, Jones MR, Rouillard AD et al (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44:W90-97. https://doi.org/10.1093/nar/gkw377
Turner SD (2014) qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv 005165. https://doi.org/10.1101/005165
Edgar RD, Jones MJ, Meaney MJ et al (2017) BECon: a tool for interpreting DNA methylation findings from blood in the context of brain. Transl Psychiatry 7:e1187. https://doi.org/10.1038/tp.2017.171
Braun PR, Han S, Hing B et al (2019) Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry 9:47. https://doi.org/10.1038/s41398-019-0376-y
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115. https://doi.org/10.1186/gb-2013-14-10-r115
Morandi L, Franceschi E, de Biase D et al (2010) Promoter methylation analysis of O6-methylguanine-DNA methyltransferase in glioblastoma: detection by locked nucleic acid based quantitative PCR using an imprinted gene (SNURF) as a reference. BMC Cancer 10:48. https://doi.org/10.1186/1471-2407-10-48
Tripathi PP, Bozzi Y (2015) The role of dopaminergic and serotonergic systems in neurodevelopmental disorders: a focus on epilepsy and seizure susceptibility. BioImpacts BI 5:97–102. https://doi.org/10.15171/bi.2015.07
Kinirons P, Verlaan DJ, Dubé M-P et al (2008) A novel locus for idiopathic generalized epilepsy in French-Canadian families maps to 10p11. Am J Med Genet A 146A:578–584. https://doi.org/10.1002/ajmg.a.32139
Wang J, Lin Z-J, Liu L et al (2017) Epilepsy-associated genes. Seizure 44:11–20. https://doi.org/10.1016/j.seizure.2016.11.030
Friedman LK, Mancuso J, Patel A et al (2013) Transcriptome profiling of hippocampal CA1 after early-life seizure-induced preconditioning may elucidate new genetic therapies for epilepsy. Eur J Neurosci 38:2139–2152. https://doi.org/10.1111/ejn.12168
Han Y, Yang L, Liu X et al (2020) HMGB1/CXCL12-Mediated Immunity and Th17 Cells Might Underlie Highly Suspected Autoimmune Epilepsy in Elderly Individuals. Neuropsychiatr Dis Treat 16:1285–1293. https://doi.org/10.2147/NDT.S242766
Zhang Y, Gao B, Xiong Y et al (2017) Expression of SHANK3 in the Temporal Neocortex of Patients with Intractable Temporal Epilepsy and Epilepsy Rat Models. Cell Mol Neurobiol 37:857–867. https://doi.org/10.1007/s10571-016-0423-7
Rakyan VK, Down TA, Thorne NP et al (2008) An integrated resource for genome-wide identification and analysis of human tissue-specific differentially methylated regions (tDMRs). Genome Res 18:1518–1529. https://doi.org/10.1101/gr.077479.108
Kobow K, Kaspi A, Harikrishnan KN et al (2013) Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathol (Berl) 126:741–756. https://doi.org/10.1007/s00401-013-1168-8
Friedman WJ (2010) Proneurotrophins, Seizures, and Neuronal Apoptosis. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 16:244–252. https://doi.org/10.1177/1073858409349903
Yang C, Shi Y, Li X et al (2022) Cadherins and the pathogenesis of epilepsy. Cell Biochem Funct 40:336–348. https://doi.org/10.1002/cbf.3699
Hodges SL, Lugo JN (2020) Therapeutic role of targeting mTOR signaling and neuroinflammation in epilepsy. Epilepsy Res 161:106282. https://doi.org/10.1016/j.eplepsyres.2020.106282
Wang Z, Ren D, Zheng P (2022) The role of Rho/ROCK in epileptic seizure-related neuronal damage. Metab Brain Dis 37:881–887. https://doi.org/10.1007/s11011-022-00909-6
Alvim MKM, Morita-Sherman ME, Yasuda CL et al (2021) Inflammatory and neurotrophic factor plasma levels are related to epilepsy independently of etiology. Epilepsia 62:2385–2394. https://doi.org/10.1111/epi.17023
Sato R, Ohmori K, Umetsu M et al (2021) An Atlas of the Quantitative Protein Expression of Anti-Epileptic-Drug Transporters, Metabolizing Enzymes and Tight Junctions at the Blood-Brain Barrier in Epileptic Patients. Pharmaceutics 13:2122. https://doi.org/10.3390/pharmaceutics13122122
Löscher W, Friedman A (2020) Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int J Mol Sci 21:E591. https://doi.org/10.3390/ijms21020591
Keck M, Androsova G, Gualtieri F et al (2017) A systems level analysis of epileptogenesis-associated proteome alterations. Neurobiol Dis 105:164–178. https://doi.org/10.1016/j.nbd.2017.05.017
van Gassen KLI, de Wit M, Koerkamp MJAG et al (2008) Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia 49:1055–1065. https://doi.org/10.1111/j.1528-1167.2007.01470.x
Chen C-M, Wang H-Y, You L-R et al (2010) Expression analysis of an evolutionarily conserved metallophosphodiesterase gene, Mpped1, in the normal and beta-catenin-deficient malformed dorsal telencephalon. Dev Dyn Off Publ Am Assoc Anat 239:1797–1806. https://doi.org/10.1002/dvdy.22293
Olson H, Shen Y, Avallone J et al (2014) Copy number variation plays an important role in clinical epilepsy. Ann Neurol 75:943–958. https://doi.org/10.1002/ana.24178
Liu Y, Zhang Y (2019) ETV5 is Essential for Neuronal Differentiation of Human Neural Progenitor Cells by Repressing NEUROG2 Expression. Stem Cell Rev Rep 15:703–716. https://doi.org/10.1007/s12015-019-09904-4
Li X, Han Y, Li D et al (2021) Identification and Validation of a Dysregulated miRNA-Associated mRNA Network in Temporal Lobe Epilepsy. BioMed Res Int 2021:1–12. https://doi.org/10.1155/2021/4118216
Guintivano J, Aryee MJ, Kaminsky ZA (2013) A cell epigenotype specific model for the correction of brain cellular heterogeneity bias and its application to age, brain region and major depression. Epigenetics 8:290–302. https://doi.org/10.4161/epi.23924
Farré P, Jones MJ, Meaney MJ et al (2015) Concordant and discordant DNA methylation signatures of aging in human blood and brain. Epigenetics Chromatin 8:19. https://doi.org/10.1186/s13072-015-0011-y
Acknowledgements
We are particularly grateful for the generous contribution of the patients and the collaboration of Biobanco-HUP (Hospital Universitario de la Princesa) and Biobank Network of the Region of Murcia, BIOBANC-MUR, registered on the Registro Nacional de Biobancos with registration number B.0000859. BIOBANC-MUR is supported by the “Instituto de Salud Carlos III (proyecto PT20/00109), by “Instituto Murciano de Investigación Biosanitaria Virgen de la Arrixaca, IMIB” and by “Consejeria de Salud de la Comunidad Autónoma de la Región de Murcia”. We would like to thank Manuel Gómez Gutierrez for his help with the study and their valuable comments on this manuscript. The genotyping was performed at the Spanish National Cancer Research Centre, in the Human Genotyping lab, a member of CeGen, PRB3 and is supported by grant PT17/0019, of the PE I+D+i 2013-2016, funded by ISCIII and ERDF. We would like to thank Dr. Agustín Fernández-Fernández and Hortensia de la Fuente for their valuable advice.
Funding
This study was supported by Instituto de Salud Carlos III: PI2017/02244. PSJ is funded by Industrial PhD grant from ‘Consejeria de Educación e Investigación’ of ‘Comunidad de Madrid’ developed in NIMGenetics and in Hospital Universitario de La Princesa (CAM.IND2017/BMD-7578).
Author information
Authors and Affiliations
Contributions
PSJ: Data curation, formal analysis, methodology, validation, and visualization. MEH and ASC: Data curation, formal analysis, methodology, and visualization. IGC: Investigation and resources. MdT, PP, MN, ABGV, MCAC, DNC, and FAS: Resources. LAG: Visualization and methodology. CVTD: Resources, project administration, funding acquisition. MCOB: Conceptualization, formal analysis, data curation, methodology, investigation, visualization, project administration, funding acquisition, supervision, and writing—original draft. All authors have read, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The protocol and the Informed Consent Form were approved by the Independent Clinical Research Ethics Committee of the Hospital Universitario de La Princesa. The study followed the STROBE guidelines and the Revised Declaration of Helsinki.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
This manuscript does not contain any individual person’s data in any form (including any individual details, images, or videos).
Conflict of Interest
F Abad-Santos has been a consultant or investigator in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Farmalíder, Ferrer, GlaxoSmithKline, Gilead, Janssen-Cilag, Kern, Normon, Novartis, Servier, Teva, and Zambon. AB Gago-Veiga has received honoraria as a consultant and speaker for: AbbVie-Allergan, Chiesi, Exeltis, Novartis, Eli Lilly, and Teva. MC Ovejero-Benito has potential conflicts of interest (honoraria for speaking and research support) with Janssen-Cilag and Leo Pharma. The rest of the authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2022_3180_MOESM1_ESM.jpg
Supplementary Fig. 1 Enrichment analysis performed with FUMA GWAS analyzing functions of the genes involved in DMPs in Cortical surrounding zone of patients compared with cortex of healthy controls. A) Biocarta. B) KEGG pathways. Red bars represent the proportion of overlapping genes in gene set. Blue bars show the enrichment p value, represented as the -logarithm of the FDR adjusted p value. Orange squares show the genes involved in every enrichment term. Abbreviation: FDR: false discovery rate. (JPG 902 KB)
12035_2022_3180_MOESM2_ESM.jpg
Supplementary Fig. 2 A) Metrics of the brain-blood correlation of the 32 differentially methylated probes observed in the hippocampus by BECon. B) Inter-individual variability of cg26834418 identified previously in blood-based studies of psychiatric disorders by BECon. C) Blood brain correlation of cg26834418 observed with IMAGE-CpG. (JPG 740 KB)
12035_2022_3180_MOESM3_ESM.jpg
Supplementary Fig. 3 An example of a differentially methylated region of the amygdala. Upper panel depicts coordinates in chromosome 3 (hg19). Orange squares represent the genes located in the chromosomic region shown. Green vertical lines show probes in the EPIC array. Differentially methylated region is shown in purple. Then, methylation values are shown for every control (green) or every patient (orange). Methylation values of every sample are shown in red or blue. Bottom panel shows methylation beta values, smoothed lines denote mean methylation levels for controls (C, forest green) and patients (T, orange). Each point represents the methylation level of a particular individual at a specific genomic location. (JPG 461 KB)
12035_2022_3180_MOESM4_ESM.jpg
Supplementary Fig. 4 An example of a differentially methylated region of the surrounding cortex to the epileptogenic zone. Upper panel depicts coordinates in chromosome 3 (hg19). Orange squares represent the genes located in the chromosomic region shown. Green vertical lines show probes in the EPIC array. Differentially methylated region is shown in purple. Then, methylation values are shown for every control (green) or every patient (orange). Methylation values of every sample are shown in red or blue. Bottom panel shows methylation beta values, smoothed lines denote mean methylation levels for controls (C, forest green) and patients (T, orange). Each point represents the methylation level of a particular individual at a specific genomic location. (JPG 487 KB)
12035_2022_3180_MOESM5_ESM.jpg
Supplementary Fig. 5 Correlation between drug resistant patients’ real age and predicted age by the epigenetic clock in the different tissues. A) Hippocampus, B) Amygdala, C) Surrounding cortex to the epileptogenic zone, D) Peripheral blood after adjusting by the different cell types. *p<0.05 (JPG 206 KB)
12035_2022_3180_MOESM7_ESM.xlsx
Supplementary Table 2. Significant differentially methylated probes associated with drug resistant epilepsy in different tissues. A) Hippocampus, B) Amygdala, C) Surrounding cortex to the epileptogenic zone. Probe location and the gene annotation were taken from Illumina reference files. * Body: Gene body; TSS1500: 1500 bp upstream of transcriptional start site (TSS): TSS200, 200bp upstream of TSS; UTR: untranslated region. %Δβ: Percentage of methylation differences between the drug resistant temporal lobe epilepsy patients and controls. chr: chromosome; FDR: false discovery rate. Probes hypomethylated in patients with respect to controls are shown in green. Probes hypermethylated in patients with respect to controls are shown in red. (XLSX 281 KB)
12035_2022_3180_MOESM8_ESM.xlsx
Supplementary Table 3. Enrichr of the significant differentially methylated probes associated with drug resistant temporal lobe epilepsy in different tissues. A) Hippocampus, B) Amygdala, C) Surrounding cortex to the epileptogenic zone. (XLSX 67.1 KB)
12035_2022_3180_MOESM9_ESM.xlsx
Supplementary Table 4. Definition of the main clusters created by Cytoscape. Cluster 1 includes 81 proteins involved in functions such as actin filament organization, growth factors and kinases. Cluster 2 is composed by 23 proteins Voltage-gated channel, and Transient receptor potential channels. Cluster 3 is formed by 20 proteins related to the spliceosome. Cluster 4 has 16 proteins involved in DNA repair. Proteins included in cluster 1 are shown in orange, cluster 2 in blue, cluster 3 in green and cluster 4 in yellow. (XLSX 174 KB)
12035_2022_3180_MOESM10_ESM.xlsx
Supplementary Table 5. Significant differentially methylated regions found in the different tissues (A-C). A) Hippocampus, B) Amygdala, C) Surrounding cortex to the epileptogenic zone. D) Enrichr of the genes located on the differentially methylated regions located in the surrounding cortex to the epileptogenic zone. Abbreviations: chr: chromosome; HMFDR: harmonic mean of the individual; meandiff: Mean differences in DNA methylation (%) between patients and controls are shown as a measurement of the effect size. Fisher <0.05 is considered significant. (XLSX 80 KB)
12035_2022_3180_MOESM11_ESM.xlsx
Supplementary Table 6. A) Number of samples from the different tissues available for the different clinical and demographic factors studied. B) Significant differentially methylated probes associated with structural etiology in different tissues. * Body: Gene body; TSS1500: 1500 bp upstream of transcriptional start site (TSS): TSS200, 200bp upstream of TSS; UTR: untranslated region. %Δβ: Percentage of methylation differences between the drug resistant epilepsy patients and controls. chr: chromosome; FDR: false discovery rate. (XLSX 13 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sánchez-Jiménez, P., Elizalde-Horcada, M., Sanz-García, A. et al. DNA Methylation Description of Hippocampus, Cortex, Amygdala, and Blood of Drug-Resistant Temporal Lobe Epilepsy. Mol Neurobiol 60, 2070–2085 (2023). https://doi.org/10.1007/s12035-022-03180-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03180-z