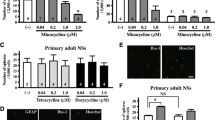
Abstract
Despite the extensive use of the cuprizone (CPZ) demyelination animal model, there is little evidence regarding the effects of CPZ on a cellular level. Initial studies have suggested that oligodendrocytes (OL) are the main cell targets for CPZ toxicity. However, recent data have revealed additional effects on neural stem cells and progenitor cells (NSC/NPC), which constitute a reservoir for OL regeneration during brain remyelination. We cultured NSC/NPC as neurospheres to investigate CPZ effects on cell mechanisms which are thought to be involved in demyelination and remyelination processes in vivo. Proliferating NSC/NPC cultures exposed to CPZ showed overproduction of intracellular reactive oxygen species and increased progenitor migration at the expense of a significant inhibition of cell proliferation. Although NSC/NPC survival was not affected by CPZ in proliferative conditions, we found that CPZ-treated cultures undergoing cell differentiation were more prone to cell death than controls. The commitment and cell differentiation towards neural lineages did not seem to be affected by CPZ, as shown by the conserved proportions of OL, astrocytes, and neurons. Nevertheless, when CPZ treatment was performed after cell differentiation, we detected a significant reduction in the number and the morphological complexity of OL, astrogliosis, and neuronal damage. We conclude that, in addition to damaging mature OL, CPZ also reduces NSC/NPC proliferation and activates progenitor migration. These results shed light on CPZ direct effects on NSC proliferation and the progression of in vitro differentiation.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47:485–505. https://doi.org/10.1016/j.neubbutiiorev.2014.10.004
Hiremath MM, Saito Y, Knapp GW, Ting JPY, Suzuki K, Matsushima GK (1998) Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol 92(1–2):38–49. https://doi.org/10.1016/S0165-5728(98)00168-4
Torkildsen O, Brunborg LA, Myhr KM, Bø L (2008) The cuprizone model for demyelination. Acta Neurol Scand Suppl 188:72–76. https://doi.org/10.1111/j.1600-0404.2008.01036.x
Norkute A, Hieble A, Braun A, Johann S, Clarner T, Baumgartner W, Beyer C, Kipp M (2009) Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J Neurosci Res 87(6):1343–1355. https://doi.org/10.1002/jnr.21946
Groebe A, Clarner T, Baumgartner W, Dang J, Beyer C, Kipp M (2009) Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum 8(3):163–174. https://doi.org/10.1007/s12311-009-0099-3
Kipp M, Clarner T, Dang J, Copray S, Beyer C (2009) The cuprizone animal model: new insights into an old story. Acta Neuropathol 118(6):723–736. https://doi.org/10.1007/s00401-009-0591-3
Buschmann JP, Berger K, Awad H, Clarner T, Beyer C, Kipp M (2012) Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. J Mol Neurosci 48(1):66–76. https://doi.org/10.1007/s12031-012-9773-x
Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M (2008) Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol 172(4):1053–1061. https://doi.org/10.2353/ajpath.2008.070850
Silvestroff L, Bartucci S, Soto E, Gallo V, Pasquini J, Franco P (2010) Cuprizone-Induced demyelination in CNP::GFP transgenic mice. J Comp Neurol 518(12):2261–2283. https://doi.org/10.1002/cne.22330
Silvestroff L, Bartucci S, Pasquini J, Franco P (2012) Cuprizone-induced demyelination in the rat cerebral cortex and thyroid hormone effects on cortical remyelination. Exp Neurol 235(2012):357–367. https://doi.org/10.1016/j.expneurol.2012.02.018
Koutsoudaki PN, Skripuletz T, Gudi V, Moharregh-Khiabani D, Hildebrandt H, Trebst C, Stangel M (2009) Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci Lett 451(1):83–88. https://doi.org/10.1016/j.neulet.2008.11.058
Gudi V, Gingele S, Skripuletz T, Stangel M (2014) Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned. Front Cell Neurosci 8:73. https://doi.org/10.3389/fncel.2014.00073
Zhang Y, Cai L, Fan K, Fan B, Li N, Gao W, Yang X, Ma J (2019) The spatial and temporal characters of demyelination and remyelination in the cuprizone animal model. Anat Rec (Hoboken) 302(11):2020–2029. https://doi.org/10.1002/ar.24216
Franklin RJ, Gilson JM, Blakemore WF (1997) Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res 50(2):337–344. https://doi.org/10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3
Gensert JM, Goldman JE (1997) Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19(1):197–203. https://doi.org/10.1016/S0896-6273(00)80359-1
El Waly B, Macchi M, Cayre M, Durbec P (2014) Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci 8:145. https://doi.org/10.3389/fnins.2014.00145
Lim DA, Alvarez-Buylla A (2014) Adult neural stem cells stake their ground. Trends Neurosci 37(10):563–571. https://doi.org/10.1016/j.tins.2014.08.006
Aguirre A, Dupree JL, Mangin JM, Gallo V (2007) A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci 10(8):990–1002. https://doi.org/10.1038/nn1938
Cayre M, Bancila M, Virard I, Borges A, Durbec P (2006) Migrating and myelinating potential of subventricular zone neural progenitor cells in white matter tracts of the adult rodent brain. Mol Cell Neurosci 31(4):748–758. https://doi.org/10.1016/j.mcn.2006.01.004
Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A (2006) Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26(30):7907–7918. https://doi.org/10.1523/JNEUROSCI.1299-06
Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Baron-Van Evercooren A (1999) Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci 11(12):4357–4366. https://doi.org/10.1046/j.1460-9568.1999.00873.x
Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van Evercooren A (2002) Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 99(20):13211–13216. https://doi.org/10.1073/pnas.192314199
Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H et al (2010) Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci 13(5):541–550. https://doi.org/10.1038/nn.2536
Ortega FB, Konstabel K, Pasquali E, Ruiz JR, Hurtig-Wennlöf A, Mäestu J, Löf M, Harro J et al (2013) Objectively measured physical activity and sedentary time during childhood, adolescence and young adulthood: a cohort study. PLoS ONE 8(4). https://doi.org/10.1371/journal.pone.0060871
Brousse B, Magalon K, Durbec P, Cayre M (2015) Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol Open 4(8):980–992. https://doi.org/10.1242/bio.012773
Butti E, Bacigaluppi M, Chaabane L, Ruffini F, Brambilla E, Berera G, Montonati C, Quattrini A et al (2019) Neural stem cells of the subventricular zone contribute to neuroprotection of the corpus callosum after cuprizone-induced demyelination. J Neurosci 39(28):5481–5492. https://doi.org/10.1523/JNEUROSCI.0227-18.2019
Skripuletz T, Gudi V, Hackstette D, Stangel M (2011) De- and remyelination in the CNS white and grey matter induced by cuprizone: The old, the new, and the unexpected. Histol Histopathol 26(12):1585–1597. https://doi.org/10.14670/HH-26.1585
Cammer W (1999) The neurotoxicant, cuprizone, retards the differentiation of oligodendrocytes in vitro. J Neurol Sci 168(2):116–120. https://doi.org/10.1016/S0022-510X(99)00181-1
Pasquini LA, Calatayud CA, Bertone Uña AL, Millet V, Pasquini JM, Soto EF (2007) The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem Res 32(2):279–292. https://doi.org/10.1007/s11064-006-9165-0
Bénardais K, Kotsiari A, Škuljec J, Koutsoudaki PN, Gudi V, Singh V, Vulinović F, Skripuletz T et al (2013) Cuprizone [bis(cyclohexylidenehydrazide)] is selectively toxic for mature oligodendrocytes. Neurotox Res 24(2):244–250. https://doi.org/10.1007/s12640-013-9380-9
Bonetto G, Charalampopoulos I, Gravanis A, Karagogeos D (2017) The novel synthetic microneurotrophin BNN27 protects mature oligodendrocytes against cuprizone-induced death, through the NGF receptor TrkA. Glia 65(8):1376–1394. https://doi.org/10.1002/glia.23170
Gómez Pinto LI, Rodríguez D, Adamo AM, Mathieu PA (2018) TGF-β pro-oligodendrogenic effects on adult SVZ progenitor cultures and its interaction with the Notch signaling pathway. Glia 66(2):396–412
Hutchins JB (1995) Platelet-derived growth factor receptors of mouse central nervous system cells in vitro. J Comp Neurol 360(1):59–80. https://doi.org/10.1002/cne.903600106
Franco PG, Pasquini JM, Silvestroff L (2015) Optimizing culture medium composition to improve oligodendrocyte progenitor cell yields in vitro from subventricular zone-derived neural progenitor cell neurospheres. PLoS ONE 10(4):1–21. https://doi.org/10.1371/journal.pone.0121774
Silvestroff L, Franco PG, Pasquini JM (2012) ApoTransferrin: dual role on adult subventricular zone-derived neurospheres. PLoS ONE 7(3):e33937. https://doi.org/10.1371/journal.pone.0033937
Silvestrof L, Franco P, Pasquini JM (2013) Neural and oligodendrocyte progenitor cells: transferrin effects on cell proliferation. ASN Neuro 5(1):e00107. https://doi.org/10.1042/AN20120075
Vega-Riquer JM, Mendez-Victoriano G, Morales-Luckie RA, Gonzalez-Perez O (2019) Five decades of cuprizone, an updated model to replicate demyelinating diseases. Curr Neuropharmacol 17(2). https://doi.org/10.2174/1570159x15666170717120343
Matsushima GK, Morell P (2001) The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol 11(1):107–116. https://doi.org/10.1111/j.1750-3639.2001.tb00385.x
Zhan J, Mann T, Joost S, Behrangi N, Frank M, Kipp M (2020) The cuprizone model: dos and do nots. Cells 9(4):843. https://doi.org/10.3390/cells9040843
Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS (2005) Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell 4(4):197–207. https://doi.org/10.1111/j.1474-9726.2005.00158.x
Zhang J, Jiao J (2015) Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed Res Int 2015:727542. https://doi.org/10.1155/2015/727542
Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M et al (2004) SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci 26(2–4):148–165. https://doi.org/10.1159/000082134
Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A (2004) Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A 101(50):17528–17532. https://doi.org/10.1073/pnas.0407893101
Alves JAJ, Barone P, Engelender S, Fróes MM, Menezes JRL (2002) Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J Neurobiol 52(3):251–265. https://doi.org/10.1002/neu.10087
Rushing G, Ihrie RA (2016) Neural stem cell heterogeneity through time and space in the ventricular-subventricular zone. Front Biol 11(4):261–284. https://doi.org/10.1007/s11515-016-1407-1
Imura T, Kornblum HI, Sofroniew MV (2003) The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci 23(7):2824–2832. https://doi.org/10.1523/JNEUROSCI.23-07-02824.2003
Jensen JB, Parmar M (2006) Strengths and limitations of the neurosphere culture system. Mol Neurobiol 34:153–161. https://doi.org/10.1385/MN:34:3:153
Reynolds BA, Weiss S (1996) Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol 175(1):1–13. https://doi.org/10.1006/dbio.1996.0090
Soares R, Ribeiro FF, Lourenço DM, Rodrigues RS, Moreira JB, Sebastião AM, Morais VA, Xapelli S (2020) Isolation and expansion of neurospheres from postnatal (P1–3) mouse neurogenic niches. J Vis Exp 159:e60822. https://doi.org/10.3791/60822
Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A (2006) PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 51(2):187–199. https://doi.org/10.1016/j.neuron.2006.06.012
Ishii Y, Matsumoto Y, Watanabe R, Elmi M, Fujimori T, Nissen J, Cao Y, Nabeshima Y et al (2008) Characterization of neuroprogenitor cells expressing the PDGF beta-receptor within the subventricular zone of postnatal mice. Mol Cell Neurosci 37(3):507–518. https://doi.org/10.1016/j.mcn.2007.11.006
Chojnacki A, Mak G, Weiss S (2011) PDGFRα expression distinguishes GFAP-expressing neural stem cells from PDGF-responsive neural precursors in the adult periventricular area. J Neurosci 31(26):9503–9512. https://doi.org/10.1523/JNEUROSCI.1531-11.2011
Gil-Perotín S, Duran-Moreno M, Cebrián-Silla A, Ramírez M, García-Belda P, García-Verdugo JM (2013) Adult neural stem cells from the subventricular zone: a review of the neurosphere assay. Anat Rec (Hoboken) 296(9):1435–1452. https://doi.org/10.1002/ar.22746
Hillis JM, Davies J, Mundim MV, Al-Dalahmah O, Szele FG (2016) Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. J Neuroinflamm 13(1):190. https://doi.org/10.1186/s12974-016-0651-2
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8(1):59–71. https://doi.org/10.1016/j.stem.2010.11.028
Morrell A, Tallino S, Yu L, Burkhead JL (2017) The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 69(4):263–270. https://doi.org/10.1002/iub.1613
Madhavan L, Ourednik V, Ourednik J (2006) Increased “vigilance” of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells 24(9):2110–2119. https://doi.org/10.1634/stemcells.2006-0018
Romanko MJ, Rothstein RP, Levison SW (2004) Neural stem cells in the subventricular zone are resilient to hypoxia/ischemia whereas progenitors are vulnerable. J Cereb Blood Flow Metab 24(7):814–825. https://doi.org/10.1097/01.WCB.0000123906.17746.00
Limoli CL, Giedzinski E, Baure J, Doctrow SR, Rola R, Fike JR (2006) Using superoxide dismutase/catalase mimetics to manipulate the redox environment of neural precursor cells. Radiat Prot Dosimetry 122(1–4):228–236. https://doi.org/10.1093/rpd/ncl458
Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schröter F, Ninnemann O, Siegert E, Bendix I et al (2008) Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol 10(4):385–394. https://doi.org/10.1038/ncb1700
Zhou D, Shao L, Spitz DR (2014) Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res 122:1–67. https://doi.org/10.1016/B978-0-12-420117-0.00001-3
Nugud A, Sandeep D, El-Serafi AT (2018) Two faces of the coin: minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. J Adv Res 14:73–79. https://doi.org/10.1016/j.jare.2018.05.012
Hwang I, Tang D, Paik J (2021) Oxidative stress sensing and response in neural stem cell fate. Free Radical Biol Med 169:74–83. https://doi.org/10.1016/j.freeradbiomed.2021.03.043
Bigarella CL, Liang R, Ghaffari S (2014) Stem cells and the impact of ROS signaling. Development 141(22):4206–4218. https://doi.org/10.1242/dev.107086
Hurd TR, DeGennaro M, Lehmann R (2012) Redox regulation of cell migration and adhesion. Trends Cell Biol 22(2):107–115. https://doi.org/10.1016/j.tcb.2011.11.002
Chiang YH, Silani V, Zhou FC (1996) Morphological differentiation of astroglial progenitor cells from EGF-responsive neurospheres in response to fetal calf serum, basic fibroblast growth factor, and retinol. Cell Transplant 5:179–189. https://doi.org/10.1016/0963-6897(95)02043-8
Brunet JF, Grollimund L, Chatton JY, Lengacher S, Magistretti PJ, Villemure JG, Pellerin L (2004) Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia 46:8–17. https://doi.org/10.1002/glia.10348
Baldassarro VA, Marchesini A, Giardino L, Calzà L (2020) Differential effects of glucose deprivation on the survival of fetal versus adult neural stem cells-derived oligodendrocyte precursor cells. Glia 68(5):898–917. https://doi.org/10.1002/glia.23750
Baur K, Abdullah Y, Mandl C, Hölzl-Wenig G, Shi Y, Edelkraut U, Khatri P, Hagenston AM et al (2022) A novel stem cell type at the basal side of the subventricular zone maintains adult neurogenesis. EMBO Rep 23(9):e54078. https://doi.org/10.15252/embr.202154078
Fernández M, Paradisi M, Del Vecchio G, Giardino L, Calzà L (2009) Thyroid hormone induces glial lineage of primary neurospheres derived from non-pathological and pathological rat brain: implications for remyelination-enhancing therapies. Int J Dev Neurosci 27(8):769–778. https://doi.org/10.1016/j.ijdevneu.2009.08.011
Covacu R, Brundin L (2016) Effects of neuroinflammation on neural stem cells. Neuroscientist 23(1):27–39. https://doi.org/10.1177/1073858415616559
Ghorbani S, Jelinek E, Jain R et al (2022) Versican promotes T helper 17 cytotoxic inflammation and impedes oligodendrocyte precursor cell remyelination. Nat Commun 13:2445. https://doi.org/10.1038/s41467-022-30032-0
Acknowledgements
We would like to thank Jimena Confessore for the artwork, Francisco Dores Piuma for support with data processing, Marianela Vence for animal care and handling, and Dr. Juana María Pasquini and Dr. Sandra Verstraeten for sharing laboratory reagents. Data available on request from the authors.
Funding
This work was supported by CONICET (Grant number PIP-CONICET 11220150100289CO to PF, PIP-CONICET 11220200101280CO to P.F.) and Universidad de Buenos Aires (UBACYT Grant number 200201190100048BA to P.F.)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yamila Azul Molinari and Agustín Jesús Byrne. The first draft of the manuscript was written by Paula Gabriela Franco and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
This study was performed in line with the principles.
All protocols in this study were approved by the local committee on animal research and ethics at Facultad de Farmacia y Bioquímica- UBA (Comité Institucional de Cuidado y Uso de Animales de Laboratorio—CICUAL) in accordance with international guidelines by Resolutions (D) Nº4538/2018 and (D) Nº 657/2020.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Suppl. Fig. 1
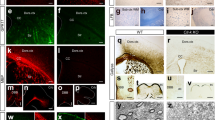
The neonatal brain as a cell source for NS cultures. a Schematic representation of a 4-day-old mouse brain adapted from Allen Developing Mouse Brain Atlas, (RRID:SCR_002990, http://developingmouse.brain-map.org/). The red box indicates the area of analysis of brain sections shown in images b-g. b-g Immunohistochemical analysis showing the expression patterns of Sox2 (b), GFAP (c), Olig2 (d), Sox10 (e), PDGFRα (f) and NG2 (g) in the selected area shown in a. NUC: Höechst stained nuclei are in blue. 200X magnification. Scale bar in d: 100 μm for images b-g. CC: corpus callosum, LV: lateral ventricle, CP: caudate putamen, CX: cortex, SVZ: subventricular zone (PNG 1015 kb)

Suppl Fig. 2
Experimental design. a Schematic view of culture protocols. Periventricular brain tissue was dissected, and cells were dissociated in B27 supplemented DMEM/F12. In the presence of bFGF and EGF, neurospheres (NS) begin to form in suspension after a few days. According to the experiment design, whole NS were attached to poly-L-lysine coated coverslips and maintained in proliferation media for migration analysis. Alternatively, NS were dissociated, and cells were plated to study the cell differentiation process in a growth factor-free medium. b Bright field view of NS in suspension, cultured for one week in proliferative conditions. c GFAP expression in NS after one day in culture after attachment to a poly-L-lysine coated surface. d Representative NS after two days in adhesion, showing GFAP expression upregulation and GFAP+ cell processes elongation. e Dissociated NS cells in proliferative conditions expressed the NSC/NPC marker Sox2 and incorporated BrdU. For differentiation studies, dissociated NS cells were cultured without growth factors. The expression of several markers for different cell types such as Sox10 (f), PDGFRα (g) and CAII (h) for oligodendroglial lineage; A2B5 (i) for glial progenitor, TujI (f) for neurons; GFAP (g) for astrocytes; and MBP (h) for mature oligodendrocytes was determined by immunofluorescence. Scale bar in b represents 100 μm, scale bar in c indicates 50 μm for c and d, and scale bar in i corresponds to 100 μm for e to i. (PNG 1530 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Molinari, Y.A., Byrne, A.J., Pérez, M.J. et al. The Effects of Cuprizone on Murine Subventricular Zone-Derived Neural Stem Cells and Progenitor Cells Grown as Neurospheres. Mol Neurobiol 60, 1195–1213 (2023). https://doi.org/10.1007/s12035-022-03096-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03096-8