Abstract
Background
An institutional management protocol for patients with subarachnoid hemorrhage (SAH) based on initial cardiac assessment, permissiveness of negative fluid balances, and use of a continuous albumin infusion as the main fluid therapy for the first 5 days of the intensive care unit (ICU) stay was implemented at our hospital in 2014. It aimed at achieving and maintaining euvolemia and hemodynamic stability to prevent ischemic events and complications in the ICU by reducing periods of hypovolemia or hemodynamic instability. This study aimed at assessing the effect of the implemented management protocol on the incidence of delayed cerebral ischemia (DCI), mortality, and other relevant outcomes in patients with SAH during ICU stay.
Methods
We conducted a quasi-experimental study with historical controls based on electronic medical records of adults with SAH admitted to the ICU at a tertiary care university hospital in Cali, Colombia. The patients treated between 2011 and 2014 were the control group, and those treated between 2014 and 2018 were the intervention group. We collected baseline clinical characteristics, cointerventions, occurrence of DCI, vital status after 6 months, neurological status after 6 months, hydroelectrolytic imbalances, and other SAH complication. Multivariable and sensitivity analyses that controlled for confounding and considered the presence of competing risks were used to adequately estimate the effects of the management protocol. The study was approved by our institutional ethics review board before study start.
Results
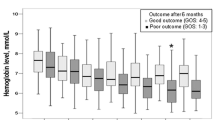
One hundred eighty-nine patients were included for analysis. The management protocol was associated with a reduced incidence of DCI (hazard ratio 0.52 [95% confidence interval 0.33–0.83] from multivariable subdistribution hazards model) and hyponatremia (relative risk 0.55 [95% confidence interval 0.37–0.80]). The management protocol was not associated with higher hospital or long-term mortality, nor with a higher occurrence of other unfavorable outcomes (pulmonary edema, rebleeding, hydrocephalus, hypernatremia, pneumonia). The intervention group also had lower daily and cumulative administered fluids compared with historic controls (p < 0.0001).
Conclusions
A management protocol based on hemodynamically oriented fluid therapy in combination with a continuous albumin infusion as the main fluid during the first 5 days of the ICU stay appears beneficial for patients with SAH because it was associated with reduced incidence of DCI and hyponatremia. Proposed mechanisms include improved hemodynamic stability that allows euvolemia and reduces the risk of ischemia, among others.

Similar content being viewed by others
References
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2012;43(6):1711–37.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
van Lieshout JH, Dibué-Adjei M, Cornelius JF, Slotty PJ, Schneider T, Restin T, et al. An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2018;41(4):917–30.
Maher M, Schweizer TA, Macdonald RL. Treatment of Spontaneous Subarachnoid Hemorrhage: Guidelines and Gaps. Stroke. 2020;1326–32.
Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93–112.
Suarez JI, Martin RH, Calvillo E, Zygun D, Flower O, Wong GK, et al. Human albumin administration in subarachnoid hemorrhage: results of an international survey. Neurocrit Care. 2014;20(2):277–86.
Suarez JI, Shannon L, Zaida OO, Suri MF, Singh G, Lynch G, et al. Effect of human albumin administration on clinical outcome and hospital cost in patients with subarachnoid hemorrhage. J Neurosurg. 2004;100(4):585–90.
Suarez JI, Martin RH, Calvillo E, Dillon C, Bershad EM, Macdonald RL, et al. The Albumin in Subarachnoid Hemorrhage (ALISAH) multicenter pilot clinical trial: safety and neurologic outcomes. Stroke. 2012;43(3):683–90.
Mayer SA, Solomon RA, Fink ME, Lennihan L, Stern L, Beckford A, et al. Effect of 5% albumin solution on sodium balance and blood volume after subarachnoid hemorrhage. Neurosurgery. 1998;42(4):758–9.
Orbegozo Cortés D, Gamarano Barros T, Njimi H, Vincent J-L. Crystalloids versus colloids: exploring differences in fluid requirements by systematic review and meta-regression. Anesth Analg. 2015;120(2):389–402.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56.
Quon CY. Clinical pharmacokinetics and pharmacodynamics of colloidal plasma volume expanders. J Cardiothorac Anesth [Internet]. 1988;2(6, Supplement 1):13–23. Available from: https://www.sciencedirect.com/science/article/pii/S0888629688800048.
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–5.
Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:1–8.
Kleinbaum, David G., Klein M. Survival Analysis—A Self-Learning Text—3rd Edition [Internet]. Springer. 2011. ii. Available from: http://linkinghub.elsevier.com/retrieve/pii/B9780444538154000248
Bhattacharyya HT, Kleinbaum DG, Kupper LL. Applied regression analysis and other multivariable methods. J Am Stat Assoc. 1979;74:732.
Austin PC, Fine JP. Accounting for competing risks in randomized controlled trials: a review and recommendations for improvement. Stat Med. 2017;36(8):1203–9.
Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–9.
Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat [Internet]. 1988 6;16(3):1141–54. Available from: http://www.jstor.org/stable/2241622.
Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45(9):1388–95.
Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–7.
Gribbons B, Herman J. True and Quasi-Experimental Designs.—Practical Assessment, Research & Evaluation. Pract Assessment, Res Eval [Internet]. 1997;5(14):3–5. Available from: http://pareonline.net/getvn.asp?v=5&n=14.
Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KTS. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br J Anaesth. 2012;109(3):315–29. https://doi.org/10.1093/bja/aes264.
Obata Y, Takeda J, Sato Y, Ishikura H, Matsui T, Isotani E. A multicenter prospective cohort study of volume management after subarachnoid hemorrhage: circulatory characteristics of pulmonary edema after subarachnoid hemorrhage. J Neurosurg. 2016;125(2):254–63.
McLaughlin N, Bojanowski MW, Girard F, Denault A. Pulmonary Edema and Cardiac Dysfunction Following Subarachnoid Hemorrhage. Can J Neurol Sci/J Can des Sci Neurol [Internet]. 2014/12/02. 2005;32(2):178–85. Available from: https://www.cambridge.org/core/article/pulmonary-edema-and-cardiac-dysfunction-following-subarachnoid-hemorrhage/EDCCB69FC8FC750E4E3A4F9304B67857.
Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P, et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med. 2018;44(4):449–63. https://doi.org/10.1007/s00134-018-5086-z.
Wang L, Li M, Xie Y, Xu L, Ye R, Liu X. Preclinical efficacy of human Albumin in subarachnoid hemorrhage. Neuroscience. 2017;344(January):255–64. https://doi.org/10.1016/j.neuroscience.2016.12.033.
Xie Y, Liu W, Zhang X, Wang L, Xu L, Xiong Y, et al. Human albumin improves long-term behavioral sequelae after Subarachnoid hemorrhage through neurovascular remodeling. Crit Care Med. 2015;43(10):e440–9.
Belayev L, Saul I, Huh PW, Finotti N, Zhao W, Busto R, et al. Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res. 1999;845(1):107–11.
Rinkel GJ, Feigin VL, Algra A, van Gijn J. Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2004;(4).
GM I, RL M. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. Neurocrit Care [Internet]. 2013;19(2):140–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23715669/.
Audibert G, Steinmann G, de Talancé N, Laurens M-H, Dao P, Baumann A, et al. Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation. Anesth Analg. 2009;108(6):1922–8.
Diringer MN, Bleck TP, Hemphill JC, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15(2):211–40.
Schwartzkopff W, Schwartzkopff B, Wurm W, Frisius H. Physiological aspects of the role of human albumin in the treatment of chronic and acute blood loss. Dev Biol Stand. 1980;48:7–30.
Anetsberger A, Gempt J, Blobner M, Ringel F, Bogdanski R, Heim M, et al. Impact of goal-directed therapy on delayed ischemia after aneurysmal subarachnoid hemorrhage: randomized controlled trial. Stroke. 2020;51(8):2287–96.
Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke. 2014;45(5):1280–4. https://doi.org/10.1161/STROKEAHA.114.004739.
Schenck H, Netti E, Teernstra O, De Ridder I, Dings J, Niemelä M, et al. The role of the glycocalyx in the pathophysiology of subarachnoid hemorrhage-induced delayed cerebral ischemia. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.731641.
Becker BF, Jacob M, Leipert S, Salmon AHJ, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80(3):389–402.
Aldecoa C, Llau JV, Nuvials X, Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020;10(1):1–12. https://doi.org/10.1186/s13613-020-00697-1.
Bell JD, Rhind SG, Di Battista AP, Macdonald RL, Baker AJ. Biomarkers of glycocalyx injury are associated with delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage: a case series supporting a new hypothesis. Neurocrit Care. 2017;26(3):339–47. https://doi.org/10.1007/s12028-016-0357-4.
Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18(5):538.
Funding
Centro de Investigaciones Clínicas, Fundación Valle del Lili (institutional research, no external funding).
Author information
Authors and Affiliations
Contributions
All authors made contributions to drafts of the manuscript and approved the final report. Conceiving the research question and developing the research protocol: JHM-M, AG, LG, AO, NJ, DR. Data collection: LG, LB, SE, AO, NJ. Statistical analysis: AG, JHM-M, MR. Interpretation of results: AG, LG, JHM-M, DR, AO, NJ, SE, LB, JEM-B.
Corresponding author
Ethics declarations
Conflict of interest
No commercial or personal relations of the authors had a conflicted interest in the development of this work.
Ethical Approval/Informed Consent
The authors confirm compliance with ethical approval; informed consent was not required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gempeler, A., Gaviria, L., Ortiz, A. et al. Effect of an Albumin Infusion Treatment Protocol on Delayed Cerebral Ischemia and Relevant Outcomes in Patients with Subarachnoid Hemorrhage. Neurocrit Care 39, 180–190 (2023). https://doi.org/10.1007/s12028-023-01731-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01731-3