Abstract
Fermented beetroots can be osmotically dehydrated and then dried to make a nutritious snack known as fermented beet chips. To ensure the best product quality, it is necessary to determine optimal parameters of osmotic dehydration. Therefore, the aim of this study was to determine the optimal parameters of pulsed vacuum osmotic dehydration (PVOD) of fermented beetroot slices. The response surface methodology (RSM) was employed to optimize PVOD and improve the efficiency of the process. The experimental parameters considered: (T) processing temperature (20 < T < 40 °C), (SC) sugar concentration (40 < SC < 60%), (VT) vacuum impregnation time (10 < VT < 50 min), and (ST) slice thickness (2 < ST < 6 mm). PVOD was optimized in terms of properties of beetroot tissue (hardness, HT, redness, a*T, water loss, WL, solid gain, SG) and osmotic solution (dry matter content, DMS, redness, a*S). The optimum qualities of beetroot tissue (HT = 202.0 N, a*T = 11.8, WL = 55.5%, SG = 7.1%) and osmotic solution (DMS = 57.3%, a*S = 38.9) were obtained at T = 20 °C, SC = 60%, VT = 10 min, ST = 6 mm and T = 40 °C, SC = 60%, VT = 50 min, ST = 2 mm, respectively. The beet tissue obtained by PVOD in optimal parameters was characterized by 2.58 ± 0.21 kg H2O/kg DM moisture content (MCF), 4.64 ± 0.37 mg GA/g DM total polyphenols (TPC), 2.2 ± 0.2 mg TE/g DM ferric reducing antioxidant power (FRAP), 1125 ± 10 kg/m3 density (ρT), and 4.29 ± 0.24 total color change (ΔE*) compared to material before PVOD. 2D and 3D images of the beetroot surface structure allowed to observe the collapse of the structure and the appearance of a semi-transparent coating (most likely a sugar solution) on the material after PVOD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beetroot (Beta vulgaris L.) is a biennial plant in the Chenopodiaceae family. The edible part of beetroot is globe or cylindrically shaped root with red–purple, golden yellow, or red-white color, depending on the variety of the beet (Chhikara et al., 2019). Beetroots are characterized by a high antioxidant capacity mostly attributed to the large amount of colorants (betalains) (Kerr & Varner, 2020). In addition, beetroots contain folic acid, vitamin C, and group B vitamins (Carrillo et al., 2019). Since beetroots increase immunity, reduce blood pressure, and prevent aging or the occurrence of cancer, they should be included into human diet (Clifford et al., 2015).
Most often, beetroots are eaten fresh or cooked, but they can also be used in the processing industry for the lactic acid fermentation to produce the beetroot leaven (Chhikara et al., 2019). While leaven is a final product of lactic acid fermentation of beetroot, fermented tissues often are considered waste. Nevertheless, this product still has a high nutritional value, and fermented beetroots can prevent and manage metabolic disorders, cardiovascular diseases, cognitive improvement, immune enhancement, etc. (Sivamaruthi et al., 2018). An interesting idea to utilize the fermented beetroots seems to be the production of fermented beetroot chips. Dried beetroot chips can be a good snack as an ideal alternative to fried potato chips. A properly selected heat source, method, and process parameters may have a significant impact on drying time and thus the properties and nutritional value of the obtained dried chips (Kudra & Mujumdar, 2009).
To partially reduce the initial amount of water in the material and thus shorten the drying time while maintaining the health-promoting properties of the product, non-thermal pre-treatment methods such as osmotic dehydration can be used (Pan et al., 2003). Osmotic dehydration can be carried out at atmospheric pressure (OD) or using a pulsed vacuum (PVOD) for a short period at the start of the process (James et al., 2014). During PVOD process, a hydrodynamic mechanism (HDM) and deformation-relaxation phenomena (DRP) take place. Due to HDM and DRP, water loss and solids gain are higher at the beginning of the PVOD compared to OD (Correa et al., 2010). In the PVOD, the most important variables are temperature, osmotic solution concentration, duration of vacuum impregnation and process time (Ito et al., 2007). However, the intensity of mass transfer during the vacuum pulse can be significantly influenced by biological characteristic of the material (Correa et al., 2010). The PVOD technique has been already applied to dehydrate fresh beetroot, carrot, eggplant, apple, cranberries, strawberries, mango, banana, and fig (Cheng et al., 2014; de Jesus Junqueira et al., 2018; Huerta-Vera et al., 2017; Ito et al., 2007; Liu et al., 2020; Macedo et al., 2021; Sahin & Ozturk, 2016; Wang et al., 2022). It was found that the application of PVOD before drying increased effective moisture diffusivity, thus shortened the drying time, and decreased shrinkage of dried figs (Sahin & Ozturk, 2016). However, the effect of vacuum on mass transfer parameters during osmotic dehydration and properties of dehydrated material is strongly related to the food microstructure (Derossi et al., 2012). For example, fresh beetroots were found to be less sensitive to the effect of PVOD than carrot or eggplant because of their less porous and more compact structure (de Jesus Junqueira et al., 2018). Additionally, fermentation is one of the treatments that can significantly affect the structure of the material. In case of table olives, fermentation caused the degradation of the skin cuticle, which facilitated polyphenols release from the tissues into the brine (Servili et al., 2008).
The intensity of the mass transfer associated with HDM and DRP during PVOD is closely related to the three-dimensional food microstructure and mechanical properties of solid matrix (Derossi et al., 2012). In order to better understand the properties and potential health benefits of fermented beetroots, it is necessary to conduct research on optimizing the PVOD of fermented beetroot. Therefore, the aim of this study was to determine the optimal parameters of PVOD of fermented beetroots in terms of dehydrated tissue as well as osmotic solution. The response surface methodology (RSM) was employed to optimize PVOD and improve the efficiency of the process. The specific objectives of this study were to: (i) investigate the effect of PVOD parameters i.e., temperature, sugar concentration in osmotic solution, duration of vacuum impregnation and slice thickness on the mass transfer parameters, redness and hardness of the beet tissue, and dry matter content and redness of the osmotic solution; (ii) fit experimental data to mathematical models; (iii) find optimum PVOD parameters; (iv) validate the estimated models, i.e., compare predicted model output to measured output; and (v) characterize the properties of PVOD products (tissue and solution).
Materials and Methods
Material
Fresh beetroots were obtained from a local supermarket (Olsztyn, Poland). The initial moisture content of fresh beetroots was estimated at 6.29 ± 0.60 kg H2O/kg DM (AOAC, 1990). The ripe vegetables were free from all diseases and were comparable in freshness and size. Before lactic acid fermentation, they were clean by a tap water and cut into 2-, 4-, and 6-mm slices.
Lactic Acid Fermentation of Beetroot
Beetroot slices (5 kg) and other spices: peppercorns (2.5 g), allspice (5.0 g), bay leaves (4.5 g), and garlic cloves (125 g) were placed together in glass jars. Then, the contents of the jars were poured with a 1.8% NaCl water solution. The sample to solution ratio was 1: 1.2. The beetroots were fermented at 20 °C for 9 days. Moisture content of the fermented beetroots was estimated at 12.45 ± 0.59 kg H2O/kg DM (AOAC, 1990). After fermentation, the beetroot slices were placed in string bags in a single layer, so that the individual slices did not touch each other. Then, the material was frozen at −18 °C (LIEBHERR GT 4932 Comfort, Bischofshofen, Austria) and kept for further processing.
Experimental Design
The experimental conditions were defined according to a Box-Behnken design. The independent variables were: temperature (T), sugar concentration in osmotic solution (SC), duration of vacuum impregnation (VT), and slice thickness (ST). In the case of osmotically dehydrated beetroot tissue, the dependent variables were as follows: hardness (HT), redness (a*T), water loss (WL), and solid gain (SG). In the case of osmotic solution, the content of dry matter (DMS) and redness (a*S) was set up as dependent variables. Matrix composed of 30 experiments was used to evaluate the optimal PVOD parameters for the material and solution. The matrix included 6 replicas at the center point to help control for variability and ensure the reliability of the PVOD results. The full experimental design was presented in Table 1.
To analyze the relationship between the independent variables and the dependent variables, a second-order polynomial equation (Eq. 1) was applied to fit the experimental data of each dependent variable:
where Y is the response variable and x corresponds to the coded variables, with the sub index i to n; a0 represents the constant of the model; ai is the coefficient of the linear terms; aii is the coefficient of the quadratic terms; aij is the coefficient of the interaction terms.
Pulsed Vacuum Osmotic Dehydration
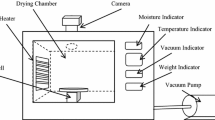
The frozen fermented beetroots were kept at room temperature for approx. 24 h to thaw before the dehydration process. Moisture content of the fermented beetroots (after thawing) was estimated at 11.79 ± 0.41 kg H2O/kg DM (AOAC, 1990). PVOD was performed in the vacuum drying oven (DZ ZBC II, Chemland, Stargard Szczecinski, Poland) under different conditions, i.e., temperature (20, 30, and 40 °C), sugar concentration in osmotic solution (40, 50, and 60%), duration of vacuum impregnation (10, 30, and 50 min), and slice thickness (2, 4, and 6 mm). Initially, the material and the osmotic solution were subjected to vacuum for 10 to 50 min, and then the samples were dehydrated at atmospheric pressure for up to 180 min. The material to solution ratio during PVOD was set at 1:4. The osmotic solution contained 5% NaCl. PVOD experiments were performed in triplicate.
Mass Transfer Parameters
The weight and the moisture content of beetroots were determined before and after PVOD. The weight was measured with an accuracy of ± 0.001 (PS 210.R2, RADWAG, Bialystok, Poland). Moisture content was determined gravimetrically using vacuum drying oven (DZ ZBC II, Chemland, Stargard Szczecinski, Poland) according to the standard (AOAC, 1990). The air temperature in the drying chamber was set at 70 °C, and heating time was 24 h. Water loss (WL) and solid gain (SG) were calculated according to the formulas presented in the literature (El-Aouar et al., 2006).
The dry matter content (DMS) of the solution was determined gravimetrically using drying oven (FED53 127, Binder, Bohemia, USA). The solution (about 5 g) was dried at 105 °C for 24 h. DMS was calculated according to the formula presented in the literature (Khajehei et al., 2015). The measurements were performed in 6 replications.
Mechanical Properties
Texture profile analysis (TPA) was performed using a texture analyzer (TA-HD plus, Stable Micro Systems, Godalming, UK). The time between compressions was 1 s, relative deformation 50% and piston speed 2 mm/s. Mechanical properties of beetroot samples were calculated using the MATLAB software (R2019a, MathWorks, Natick, USA). Such properties as hardness (H), cohesiveness (C), springiness (Sp), gumminess (G), and chewiness (Ch) were obtained from the force–deformation curve (Zielinska et al., 2015). Each sample was analyzed in 15 repetitions.
Color
Beetroot tissue and solution color measurements were carried out using a spectrophotometer (3Color 9000Neo, TRI-COLOR, Narama, Poland) in reflection mode under standard D65 illumination, 10° observer, and 18 mm diaphragm. Results were expressed in the CIEL*a*b* space. The indices—ΔE* (total color difference), ΔC* (total saturation difference), and ΔH* (total hue difference)—were calculated according to the formulas presented in the literature (Zielinska & Markowski, 2012). The results for beetroot tissue and osmotic solution were averaged over 30 and 6 measurements, respectively.
Density
Density of osmotic solution was determined by pycnometric method using demineralized water as a reference. Density of beetroot tissue was determined by the hydrostatic method, i.e., weighing the sample in air and in demineralized water. The weight was measured with an accuracy of ± 0.001 (PS 210.R2, RADWAG, Bialystok, Poland). Density measurements of osmotic solution and beetroot tissue were performed at room temperature (20 ± 1 °C) in 3 and 5 repetitions, respectively.
Dynamic Viscosity of Osmotic Solution
The dynamic viscosity (η) of the osmotic solution was determined using a rotary rheometer (Rheotest 2.1, VEB MLW, Medingen, Germany). Based on tangential stress values measured at selected shear rates in the range from 0.03 to 1300 1/s, the flow curves were plotted. The measuring chamber of the rheometer was surrounded with a water jacket with thermo-circulation, which helped to maintain the required measurement temperature (t = 20 °C). The measurements were performed in triplicate.
Viscoelastic Properties of Beetroot Tissue
The rheological properties of fermented beetroot tissue were evaluated by stress relaxation test using Texture Analyzer (TA.HD plus, Stable Micro Systems Ltd., Godalming, UK). The strain and duration set for the test were 20% and 60 s, respectively. Slices with a diameter of 12.5 mm and a thickness of 6 mm were used in the experiments. The parameters of a three-element Maxwell model (Eq. 2) were estimated based on stress relaxation curves over time domain, utilizing Excel Solver as a tool (MS Excel, Microsoft, Washington, USA):
where σ is stress [Pa]; ε0 is initial deformation [-]; E is stiffness modulus [Pa]; t is time [s]; and τR is relaxation time [s].
Subsequently, the summed stiffness modulus (ΣE, as the sum of E0, E1, E2, and E3) and mean relaxation time (τRM, as the mean value of τR1, τR2, and τR3) were calculated. The measurements were performed in 12 repetitions.
Surface Images
Digital image microscopy was used to capture 2D and 3D surface images of the beetroot tissue before and after PVOD treatment at magnification of × 20, × 100, and × 500. The images were obtained using digital microscope with dedicated software (Keynes VHX 7000, Mechelen, Belgium) and captured at 4 k resolution using at full illumination system.
Total Phenolics and Antioxidant Activity by Spectrophotometric Method
Bioactive compounds were extracted from a pulverized dried sample (approx. 0.05 g) by sonication (VC 750, Sonics & Materials, Newtown, CT, USA) with 1 mL of acidified 80% methanol (0.1% HCl: v/v) for 30 s. The extraction procedure has been described in detail in the literature (Zielinska & Zielinska, 2019). The extraction was performed in triplicate.
The total phenolics content (TPC) and ferric reducing antioxidant power (FRAP) were determined according to the procedure described by Zielinska and Zielinska (Zielinska & Zielinska, 2019). The TPC and FRAP values were expressed as mg of gallic acid (mg GA) and Trolox (mg TE) equivalents per g of dry matter (g DM), respectively. All measurements were performed in triplicate.
Statistical Analyses
Statistical analyses were performed using the STATISTICA 13.0 software (TIBCO Software Inc., Palo Alto, CA, USA). Regression analysis and analysis of variance (ANOVA) were performed to fit the models represented by the Eq. 1 and to examine the statistical significance of model conditions. Only significant model conditions were included into the final version (p < 0.05). To assess the quality of the model fit to the experimental data, two metrics, i.e., the coefficient of determination (R2) and percentage global relative square error of the approximation (GRSE) were utilized. The lowest values (nearest to zero) of GRSE and the highest values of R2 (> 0.90) indicated the best fit of the model to the experimental data (Staniszewska et al., 2022). Correlations (r) between the independent and dependent variables were analyzed using the Pearson correlation coefficient test.
Optimization and Validation of the Process Conditions
Optimization of PVOD was performed using the STATISTICA 13.0 software (TIBCO Software Inc., Palo Alto, CA, USA). The aim of optimization was to determine the appropriate level of the four factors (temperature, sugar concentration in osmotic solution, duration of vacuum impregnation, and slice thickness) that would result in (1) the highest a*T and WL and the lowest HT and SG for dehydrated beetroot tissue and (2) the highest a*S and DMS for osmotic solution.
To validate the predicted process conditions, an experiment was conducted using the optimized conditions, and the predicted values from the models were compared to the actual values obtained during the experiment. The relative error (RE) was used to compare the experimental and predicted values. RE was calculated according to the formula given in the literature (Gao et al., 2006).
Results and Discussion
Model Fitting
The design of experiments, including levels of four independent variables and actual responses for each combination, is presented in Table 1. Experimental responses related to the beetroot tissue, i.e., hardness (HT), redness (a*T), water loss (WL), solid gain (SG), and final moisture content of the material (MCF) varied from 212 to 549 N, from 8.4 to 16.6, from 34.5 to 65.4%, from 6.2 to 23.1%, and from 1.42 ± 0.21 to 2.74 ± 0.30 kg H2O/kg DM, respectively. The experimental responses for the dry matter content of osmotic solution (DMS) and redness (a*S) ranged from 38.1 to 58.3% and from 30.2 to 44.0, respectively (Table 1). A second-order polynomial equation (Eq. 1) was fitted based on the experimental data. Values of full model parameters, as well as t- and p-values of each estimated regression coefficients, are presented in Table 2.
The high determination coefficients (0.97 > R2 > 0.72) indicate that obtained quadratic models well described influences of independent variables on the analyzed responses. However, analysis of variance (ANOVA) and calculated p-values indicated that not all of the model terms were significant. Therefore, in relation to the analyzed dependent variables, the full forms of the models were reduced by non-significant coefficients (p > 0.05). Term reduction was simultaneously evaluated by R2 and GRSE. Simplified models of dependent variables were as follows:
The values of R2 and GRSE of the reduced models varied from 0.75 to 0.98 and from 2.5 to 18.1, respectively (Eqs. 3–8). The results indicate good correlation (0.98 > r > 0.79) between the actual and predicted values (Fig. 1). It can be noticed, especially in the case of SG and a*S, that the simplification of the models allowed to obtain higher fit coefficients.
The highest correlations were found between the dependent variables and process parameters, i.e., between DMS vs SC (r = 0.97), HT vs ST (r = −0.81), WL vs SC (r = 0.79), a*S vs ST (r = −0.64), a*T vs ST (r = −0.62), and SG vs ST (r = −0.61). These correlations were reflected in the significance of the regression coefficients. The regression coefficients of the models with the dependent variables that were most correlated to the independent variables were found to be the most significant or among the most significant (p = 0.001) (Table 2).
Influence of Process Variables on the Responses
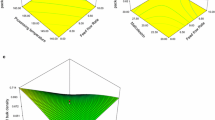
Response surface plots for variables predicted by reduced models are presented in Fig. 2. As can be seen, slice thickness (ST) was the most influential process condition, and it was a significant factor in all output variables studied.
Response surface plots for the following: a HT in relation to ST and SC; b a*T in relation to VT and ST; c WL in relation to ST and SC; d SG in relation to T and ST; e DMS in relation to ST and SC, and f a*S in relation to ST and SC. Conditions of other factors were kept constant at their central values, i.e., T at 30 °C, SC at 50%, VT at 30 min, and ST at 4 mm. Symbols: T, processing temperature, °C; SC, sugar concentration in osmotic solution, %; VT, vacuum impregnation time, min; ST, slice thickness, mm; H, hardness, N; a*, redness, -; WL, water loss, %; SG, sugar gain, %; DM, dry matter content, %. Subscripts: T, beetroot tissue; S, osmotic solution
Hardness of Tissue
The modeled value of hardness of beetroot tissue (HT) after PVOD varied from 202 to 463 N (Fig. 2a). The linear negative significant effect of slice thickness (ST) on HT was observed (Table 2), and the value of linear regression coefficient was −110.79 (Eq. 3). This suggests that the hardness of the tissue increased as the slice thickness decreased (Fig. 2a). This can be related to the significant linear negative influence of slice thickness (ST) on the solid gain (SG) during PVOD (Eq. 6). The dry matter content in thinner samples could lead to a direct increase in their hardness (Zhao et al., 2014).
At the quadratic level, the most significant parameter of the process affecting the HT was sugar concentration of the osmotic solution (SC) (Table 2). The value of the quadratic regression coefficient related to this independent variable was -39.57 (Eq. 3).
Redness of Tissue
After PVOD, the predicted redness of beetroot tissue (a*T) varied from 9.6 to 16.5 (Fig. 2b). Table 2 indicates that the process temperature (T) was the only process variable that did not have a significant effect (p < 0.05) on the a*T value. Based on the linear regression coefficients of reduced model, it can be stated that slice thickness (ST) (−1.94) followed by vacuum time (VT) (−1.27) and solid concentration (SC) (−0.76) had the most significant, negative effect on the redness of beetroots (Eq. 4). Higher retention of red pigments at lower solid concentration could result from the lower diffusion coefficient of water-soluble betalains into the solution (de Jesus Junqueira et al., 2018).
Betalains, which are water-soluble pigments responsible for red color in many plants, have also a lower diffusion coefficient in water compared to other pigments. Therefore, at lower solid concentration, there is a slower diffusion of betalains into the solution, resulting in a higher retention of red pigments in the plant tissue.
However, vacuum time (VT) and slice thickness (ST) were also significant parameters at the quadratic level and their effect was opposite to that found for the linear terms (Table 2). This indicate that only the influence of sugar concentration (SC) on the redness of beet tissue can be interpreted unequivocally as linear and negative, while the influence of other significant variables was found to be more complex and not obvious in interpretation.
Water Loss of Tissue
Predicted water loss (WL) during PVOD was from 33.6 to 64.8% (Fig. 2c). The most significant linear regression coefficient was sugar concentration (SC) and it was 10.04 (Table 2, Eq. 5). In the reduced model, slice thickness (ST) at the quadratic level was the only significant regression coefficient and its value was -5.87 (Eq. 5). The results indicate the increasing nature of water loss (WL) along with the increasing sugar concentration (SC), and the highest WL at solid concentration (ST) of 4 mm (at each SC).
Solid Gain of Tissue
The values of solid gain (SG) predicted by the reduced models were in range from 7.0 to 21.7% (Fig. 2d). Slice thickness (ST) was found to have the most significant negative linear influence (−4.37) on the solid gain of beetroot tissues (Eq. 6). This could be due to less penetration of sugar inside the deeper part through osmosis during PVOD. The results are consistent with the data published for instant ginger candy (Nath et al., 2013). The slice thickness (ST) at the quadratic level (Table 2) also had a significant effect on the solid gain (SG) of beetroot tissues and the square regression coefficient of SG was 2.66 (Eq. 6). The process temperature (T) showed a linear positive effect (2.35) on solid gain (SG) (Eq. 6). Similar effect of temperature (T) on the solid gain (SG) during osmotic dehydration of potato and apple was described in the literature (Eren & Kaymak-Ertekin, 2007; Sereno et al., 2001).
Dry Matter Content of Solution
The predicted content of dry matter of the osmotic solution (DMS) ranged from 38.8 to 57.3% (Fig. 2e). Solution concentration (SC) had significant linear and slice thickness (ST) significant quadratic positive effect on DMS (Table 2, Eq. 7). The quadratic positive effect of slice thickness (ST) on the degree of moisture loss (DMS) could be strongly related to the fact that slice thickness has a significant quadratic influence on water loss (WL) in a negative way (Eq. 5). Thus, samples with a slice thickness of 4 mm exhibited the highest water loss (WL) during PVOD, leading to a lower percentage of dry matter content in the osmotic solution (Figs. 2c, e).
Redness of Solution
The modeled redness of the osmotic solution (a*S) ranged from 31.5 to 42.9 (Fig. 2f). It is noticeable that the lowest and highest values of a*S were predicted at the same temperature (T) and vacuum time (VT) and different solid concentration (SC) and slice thickness (ST). This can be attributed to the significant effect of SC and ST on a*S, where SC had a linear and negative impact, while ST had both a linear negative and quadratic positive impact (Table 2, Eq. 8).
Optimization of the Process Conditions
The optimal parameters of the PVOD process were valid only in the selected experimental domain, which was in the range 20–40 °C of T, 40–60% of SC, 10–50 min of VT, and 2–6 mm of ST. Minimization of HT and SG and maximization of a*T and WL were used as criteria in optimization of PVOD of beetroot tissue. The optimized values were found to be T = 20 °C, SC = 60%, VT = 10 min, and SC = 6 mm. The predicted HT, a*T, WL, and SG values under optimum conditions were 202 N, 11.8, 55.5%, and 7.1%, respectively. The maximization of DMS and a*S was criterion in optimization of PVOD of osmotic solution. In this case, the optimal process parameters were as follows: T = 40 °C, SC = 60%, VT = 50 min, and ST = 2 mm. With the optimal process parameters determined through this approach, the predicted DMS and a*S values were 57.3% and 38.9, respectively.
Model Validation
The model validation was carried out using the optimal PVOD parameters that were determined through the optimization approach that utilized beetroot tissue properties, i.e., T = 20 °C, SC = 60%, VT = 10 min, and ST = 6 mm. According to the regression models, the predicted values of HT, a*T, WL, SG, DMS, and a*S under above conditions were 202 N, 11.8, 55.5%, 7.1%, 57.3%, and 31.5, respectively (Table 3). Table 3 shows that the values of the output variables were deemed satisfactory based on the relative error (RE), which ranged from 1 to 3%.
Characterization of PVOD Products
PVOD of fermented beetroot slices carried out under optimal parameters allowed to reduce the moisture content of beetroot tissue by 78%, i.e., from 11.79 ± 0.41 to 2.58 ± 0.21 kg H2O/kg DM. During PVOD, the TPC and FRAP of beetroot tissue decreased by 48 and 83%. At the same time, TPC and FRAP in the osmotic solution increased and amounted to 49.2 ± 9.6 mg GA/g DM and 204 ± 12 mg TE/g DM, respectively (Table 4). The main mechanism responsible for the reduction of phenolic compounds in beetroot tissue during the PVOD was water diffusion. It can be stated that water-soluble phenols can be leached out through the water flow from the plant material into the surrounding osmotic solution (Nicetin et al., 2022).
PVOD caused more significant changes in color parameters (L*, a*, b*) and color indices (ΔE*, ΔC*, ΔH*) of osmotic solution than beetroot tissue (Table 4). This was probably due to the leaching of other water-soluble compounds, such as betalains, which are a natural pigments of beetroot tissue (de Jesus Junqueira et al., 2018). Due to the transparent color of the solution before dehydration, the addition of these compounds caused a significant change in color.
PVOD also had a significant effect on mechanical and rheological properties of beetroot tissue (Table 4). During processing, hardness of the material decreased from 333 ± 15 to 197 ± 15 N. These results are consistent with literature data (Pei et al., 2023). Other mechanical properties, such as cohesiveness, chewiness, gumminess, and springiness also increased during processing (Table 4). The mean values of rheological parameters of beetroot tissue such as ΣE and τRM show that the tissue was less stiff and resilient, and more liable for stress relaxation after osmotic dehydration (Table 4).
During PVOD, density of the beetroot tissue increased by 9%, while density of osmotic solution decreased by 4%. This was due to the moisture diffusion from the material into the solution and solids (sugar) from the solution into the material. Moreover, due to dilution of the osmotic solution, the dynamic viscosity of the osmotic solution decreased by 75% from 135 ± 1 to 35 ± 1 mPa⋅s (Table 4).
The influence of PVOD on the surface structure of the beetroot tissue was presented in Fig. 3. A significant change in the maximum relative height of the sample (the difference between the highest and lowest point in the examined area) can be found in the 3D images of samples at each given magnification. For example, the maximum relative height of the beetroot tissue before PVOD was 938 μm (Fig. 3a, magnification of × 20), while after PVOD, the height increases up to 2026 μm (Fig. 3d, magnification of × 20). Most likely, the difference was related to the water loss from beetroot tissue during processing, which led to volume reduction and material shrinkage. Before PVOD, highly porous structure of the surface of beetroot tissue was clearly presented using 2D images of samples (Fig. 3a, b). After osmotic dehydration, semi-transparent layer of sugar solution coated the surface of beetroot tissue, providing additional barrier to moisture removal during subsequent drying processes (Fig. 3e, f).
Conclusions
Pulsed vacuum osmotic dehydration (PVOD) of fermented beetroots was optimized in terms of properties of both beetroot tissue (HT, a*T, WL, SG) and osmotic solution (DMS, a*S). Response surface methodology was utilized to optimize PVOD. The experimental parameters considered: (T) processing temperature (20 < T < 40 °C), (SC) sugar concentration in osmotic solution (40 < SC < 60%), (VT) vacuum impregnation time (10 < VT < 50 min), and (ST) slice thickness (2 < ST < 6 mm). The optimum properties of beetroot tissue (HT at 202 N, a*T at 11.8, WL at 55.5%, SG at 7.1%) and osmotic solution (DMS at 57.3%, a*S at 38.9) were obtained for T = 20 °C + SC = 60% + VT = 10 min + ST = 6 mm and T = 40 °C + SC = 60% + VT = 50 min + ST = 2 mm, respectively.
PVOD caused a significant physicochemical, mechanical, rheological, and color changes in both beetroot tissue and osmotic solution. During PVOD, the TPC and FRAP values of beetroot tissue decreased by 48 and 83%, while TPC and FRAP values of solution increased up to 49.2 ± 9.6 mg GA/g DM and 204 ± 12 mg TE/g DM. It was due to the leaching of water-soluble bioactive compounds from the material into the surrounding solution. Also, migration of bioactive compounds from the material to the solution caused a significant total color change, i.e., 39.1 ± 0.3 and 4.29 ± 0.24 for the solution and beetroot tissue, respectively. Due to the moisture diffusion during PVOD, the density of the material increased from 1034 ± 5 to 1125 ± 10 kg/m3, while the density of the osmotic solution decreased from 1336 ± 1 to 1280 ± 1 kg/m3. Dilution of the osmotic solution also caused the decrease of its viscosity by 75%, i.e., from 135 ± 1 to 35 ± 1 mPa⋅s. 2D and 3D images of the beetroot surface structure allow observation of the collapse of the structure and the appearance of a semi-transparent coating (most likely a sugar solution) on the material after PVOD. This coating may provide an additional barrier to water removal during subsequent drying.
The optimal PVOD conditions of fermented beetroot slices determined in this work may be beneficial for producers of fermented products for managing of post-fermentation products. In future works, it would be interesting to carry out PVOD processes without the freezing stage, which may affect the structure of the material, and thus the kinetics of the process. Also, it would be interesting to explore non-thermal methods, such as ultrasound, to assist with PVOD and investigate their impact on mass transfer during the process and the quality of fermented beetroot slices.
Data Availability
The datasets generated during the current study are available from the corresponding author on request.
References
AOAC. (1990). Official method of analysis. Association of official analytical chemists. Arlington, VA, (No. 934.06)
Carrillo, C., Wilches-Perez, D., Hallmann, E., Kazimierczak, R., & Rembialkowska, E. (2019). Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. LWT-Food Science and Technology, 116, 108552. https://doi.org/10.1016/j.lwt.2019.108552
Cheng, X. F., Zhang, M., Adhikari, B., & Islam, N. (2014). Effect of power ultrasound and pulsed vacuum treatments on the dehydration kinetics, distribution, and status of water in osmotically dehydrated strawberry: A combined NMR and DSC study. Food and Bioprocess Technology, 7, 2782–2792. https://doi.org/10.1007/s11947-014-1355-1
Chhikara, N., Kushwaha, K., Sharma, P., Gat, Y., & Panghal, A. (2019). Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chemistry, 272, 192–200. https://doi.org/10.1016/j.foodchem.2018.08.022
Clifford, T., Howatson, G., West, D., & Stevenson, E. (2015). The potential benefits of red beetroot supplementation in health and disease. Nutrients, 7(4), 2801–2822. https://doi.org/10.3390/nu7042801
Correa, J. L. G., Pereira, L. M., Vieira, G. S., & Hubinger, M. D. (2010). Mass transfer kinetics of pulsed vacuum osmotic dehydration of guavas. Journal of Food Engineering, 96(4), 498–504. https://doi.org/10.1016/j.jfoodeng.2009.08.032
de Jesus Junqueira, J. R., Correa, J. L. G., de Mendonca, K. S., de Mello Junior, R. E., & de Souza, A. U. (2018). Pulsed vacuum osmotic dehydration of beetroot, carrot and eggplant slices: Effect of vacuum pressure on the quality parameters. Food and Bioprocess Technology, 11(10), 1863–1875. https://doi.org/10.1007/S11947-018-2147-9/FIGURES/4
Derossi, A., De, T., & Severini, C. (2012). The application of vacuum impregnation techniques in food industry. In Scientific, Health and Social Aspects of the Food Industry. Institute for New Technologies. https://doi.org/10.5772/31435
El-Aouar, A.A., Azoubel, P.M., Barbosa, J.L., & Xidieh Murr, F.E. (2006). Influence of the osmotic agent on the osmotic dehydration of papaya (Carica papaya L.). Journal of Food Engineering, 75(2), 267–274. https://doi.org/10.1016/j.jfoodeng.2005.04.016
Eren, I., & Kaymak-Ertekin, F. (2007). Optimization of osmotic dehydration of potato using response surface methodology. Journal of Food Engineering, 79(1), 344–352. https://doi.org/10.1016/j.jfoodeng.2006.01.069
Gao, N., Zhu, S. A., & He, B. (2006). A new magnetic resonance electrical impedance tomography (MREIT) algorithm: The RSM-MREIT algorithm with applications to estimation of human head conductivity. Physics in Medicine and Biology, 51(12), 3067–3083. https://doi.org/10.1088/0031-9155/51/12/005
Huerta-Vera, K., Flores-Andrade, E., Perez-Sato, J. A., Morales-Ramos, V., Pascual-Pineda, L. A., & Contreras-Oliva, A. (2017). Enrichment of banana with Lactobacillus rhamnosus using double emulsion and osmotic dehydration. Food and Bioprocess Technology, 10, 1053–1062. https://doi.org/10.1007/s11947-017-1879-2
Ito, A. P., Tonon, R. V., Park, K. J., & Hubinger, M. D. (2007). Influence of process conditions on the mass transfer kinetics of pulsed vacuum osmotically dehydrated mango slices. Drying Technology, 25(10), 1769–1777. https://doi.org/10.1080/07373930701593263
James, C., Purnell, G., & James, S. J. (2014). A critical review of dehydrofreezing of fruits and vegetables. Food and Bioprocess Technology, 7, 1219–1234. https://doi.org/10.1007/s11947-014-1293-y
Kerr, W. L., & Varner, A. (2020). Chemical and physical properties of vacuum-dried red beetroot (Beta vulgaris) powders compared to other drying methods. Drying Technology, 38(9), 1165–1174. https://doi.org/10.1080/07373937.2019.1619573
Khajehei, F., Niakousari, M., Eskandari, M. H., & Sarshar, M. (2015). Production of pomegranate juice concentrate by complete block cryoconcentration process. Journal of Food Process Engineering, 38(5), 488–498. https://doi.org/10.1111/jfpe.12179
Kudra, T., & Mujumdar, A. S. (2009). Advanced drying technologies (2nd ed.). CRC Press.
Liu, Z. L., Staniszewska, I., Zielinska, D., Zhou, Y. H., Nowak, K. W., Xiao, H. W., Pan, Z., & Zielinska, M. (2020). Combined hot air and microwave-vacuum drying of cranberries: Effects of pretreatments and pulsed vacuum osmotic dehydration on drying kinetics and physicochemical properties. Food and Bioprocess Technology, 13, 1848–1856. https://doi.org/10.1007/s11947-020-02507-9
Macedo, L. L., Correa, J. L. G., da Silva Araujo, C., Vimercati, W. C., & Junior, I. P. (2021). Convective drying with ethanol pre-treatment of strawberry enriched with isomaltulose. Food and Bioprocess Technology, 14, 2046–2061. https://doi.org/10.1007/s11947-021-02710-2
Nath, A., Deka, B. C., Jha, A. K., Paul, D., & Misra, L. K. (2013). Effect of slice thickness and blanching time on different quality attributes of instant ginger candy. Journal of Food Science and Technology, 50(1), 197–202. https://doi.org/10.1007/S13197-012-0619-X/FIGURES/3
Nicetin, M., Pezo, L., Pergal, M., Loncar, B., Filipovic, V., Knezevic, V., Demir, H., Filipovic, J., & Manojlovic, D. (2022). Celery root phenols content, antioxidant capacities and their correlations after osmotic dehydration in molasses. Foods, 11(13), 1945. https://doi.org/10.3390/foods11131945
Pan, Y. K., Zhao, L. J., Zhang, Y., Chen, G., & Mujumdar, A. S. (2003). Osmotic dehydration pretreatment in drying of fruits and vegetables. Drying Technology, 21(6), 1101–1114. https://doi.org/10.1081/DRT-120021877
Pei, Y. P., Sun, B. H., Vidyarthi, S. K., Zhu, Z. Q., Yan, S. K., Zhang, Y., Wang, J., & Xiao, H. W. (2023). Pulsed pressure enhances osmotic dehydration and subsequent hot air drying kinetics and quality attributes of red beetroot. Drying Technology, 41(2), 262–276. https://doi.org/10.1080/07373937.2022.2031209
Sahin, U., & Ozturk, H. K. (2016). Effects of pulsed vacuum osmotic dehydration (PVOD) on drying kinetics of figs (Ficus carica L). Innovative Food Science and Emerging Technologies, 36, 104–111. https://doi.org/10.1016/j.ifset.2016.06.003
Sereno, A. M., Moreira, R., & Martinez, E. (2001). Mass transfer coefficients during osmotic dehydration of apple in single and combined aqueous solutions of sugar and salt. Journal of Food Engineering, 47(1), 43–49. https://doi.org/10.1016/S0260-8774(00)00098-4
Servili, M., Minnocci, A., Veneziani, G., Taticchi, A., Urbani, S., Esposto, S., Sebastiani, L., Valmorri, S., & Corsetti, A. (2008). Compositional and tissue modifications induced by the natural fermentation process in table olives. Journal of Agricultural and Food Chemistry, 56(15), 6389–6396. https://doi.org/10.1021/jf8007019
Sivamaruthi, B., Kesika, P., Prasanth, M., & Chaiyasut, C. (2018). A mini review on antidiabetic properties of fermented foods. Nutrients, 10(12), 1973. https://doi.org/10.3390/nu10121973
Staniszewska, I., Dzadz, L., Xiao, H. W., & Zielinska, M. (2022). Evaluation of storage stability of dried cranberry powders based on the moisture sorption isotherms and glass transition temperatures. Drying Technology, 40(1), 89–99. https://doi.org/10.1080/07373937.2020.1771362
Wang, X., Kahraman, O., & Feng, H. (2022). Impact of osmotic dehydration with/without vacuum pretreatment on apple slices fortified with hypertonic fruit juices. Food and Bioprocess Technology, 15, 1588–1602. https://doi.org/10.1007/s11947-022-02834-z
Zhao, J. H., Hu, R., Xiao, H. W., Yang, Y., Liu, F., Gan, Z. L., & Ni, Y. Y. (2014). Osmotic dehydration pretreatment for improving the quality attributes of frozen mango: Effects of different osmotic solutes and concentrations on the samples. International Journal of Food Science and Technology, 49, 960–968. https://doi.org/10.1111/ijfs.12388
Zielinska, M., & Markowski, M. (2012). Color characteristics of carrots: Effect of drying and rehydration. International Journal of Food Properties, 15(2), 450–466. https://doi.org/10.1080/10942912.2010.489209
Zielinska, M., & Zielinska, D. (2019). Effects of freezing, convective and microwave-vacuum drying on the content of bioactive compounds and color of cranberries. LWT-Food Science and Technology, 104, 202–209. https://doi.org/10.1016/j.lwt.2019.01.041
Zielinska, M., Sadowski, P., & Blaszczak, W. (2015). Freezing/thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.). LWT-Food Science and Technology, 62(1), 555–563. https://doi.org/10.1016/J.LWT.2014.08.002
Acknowledgements
We would like to thank Mr. Bartosz Pszczolkowski and Mr. Lukasz Dzadz for their technical support.
Funding
This study was supported by the National Science Centre in Poland (grant No. 2020/37/B/NZ9/00687, title: The effect of ultrasound, microwaves, infrared radiation and reduced pressure on the dehydration and drying kinetics of beetroots and the University of Warmia and Mazury in Olsztyn (grant No. 16.610.001–110).
Author information
Authors and Affiliations
Contributions
Izabela Staniszewska: conceptualization, investigation, formal analysis, validation, visualization, methodology, writing—original draft and editing, writing—review and editing; Konrad W. Nowak: formal analysis, investigation, writing—original draft and editing; Danuta Zielinska: methodology, substantive support, writing—original draft and editing; Iwona Konopka: conceptualization, methodology, writing—original draft and editing; Magdalena Zielinska: conceptualization, methodology, writing—original draft and editing, resources, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Staniszewska, I., Nowak, K.W., Zielinska, D. et al. Pulsed Vacuum Osmotic Dehydration (PVOD) of Fermented Beetroot: Modeling and Optimization by Response Surface Methodology (RSM). Food Bioprocess Technol 17, 977–990 (2024). https://doi.org/10.1007/s11947-023-03173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03173-3