Abstract
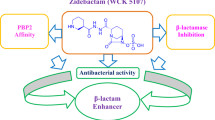
Zidebactam (WCK 5107) is a novel β-lactam enhancer (BLE), developed by Wockhardt, exhibiting standalone antimicrobial activities against panel of gram-negative pathogens. Zidebactam is referred as β-lactam ‘enhancer’, because in combination with a partner β-lactam drug, zidebactam not only inhibits the β-lactamases, but also targets PBP2 and enhances the potency of partner drug. The manuscript details the stereoselective synthesis of Zidebactam, as well as the technique/process for preparing the stable sodium salt of (2S,5R)-6-benzyloxy-7-oxo-1,6-diaza-bicyclo[3.2.1]-octane-2-carboxylic acid and the chirally pure side chain, N-Boc-(R)-(-)-ethyl nipecotate hydrazide via chiral resolution. The convergent synthesis of zidebactam has achieved by coupling between sodium salt of DBO carboxylic acid and Boc-(R)-(-)-ethyl nipecotate hydrazide followed by requisite chemical transformations. Single crystal X-ray analysis established the stereochemistry of zidebactam with empirical formula, C13H21N5O7S.2H2O. Currently, two drug combination products zidebactam with cefepime (WCK 5222) and zidebactam with ertapenem (WCK 6777) awarded qualified infectious disease product (QIDP) status by U.S. Food and Drug Administration (USFDA). WCK 5222 has completed phase I clinical trial studies in the USA and is currently undergoing global phase III clinical studies, WCK 6777 is progressing through phase I clinical studies.
Graphical Abstract












Similar content being viewed by others
References
Ahirrao V, Rane V, Patil K, Jadhav R, Bhamre V, Yadav D, Yeole R (2020) Identification of mono-methyl sulfate and sulfate impurities in Zidebactam using LC-MS and application of mixed-mode liquid chromatography with charged aerosol detection and ion chromatography for quantification. Chromatographia 83:219–228. https://doi.org/10.1007/s10337-019-03836-4
Akova M (2008) Sulbactam-containing beta-lactamase inhibitor combinations. Clin Microbiol Infect 14(Suppl 1):185–188. https://doi.org/10.1111/j.1469-0691.2007.01847.x
Bhavsar S, Joshi S, Pawar S, Pavase L, Mishra A, Jadhav S, Dabhade S, Kayastha AK, Yeole R, Deshpande P, Bhagwat S, Patel M et al (2023) Structure activity relationship (SAR) driven design and discovery of WCK 5107 (Zidebactam): novel β-lactam enhancer, potent against multidrug-resistant Gram negative pathogens. Med Chem Res 24:456. https://doi.org/10.1007/s00044-023-03135-6
Bouza E (2021) The role of new carbapenem combinations in the treatment of multidrug-resistant gram-negative infections. J Antimicrob Chemother 76(Suppl 4):38–45. https://doi.org/10.1093/jac/dkab353
Cohen JH, Bos ME, Cesco-Cancian S et al (2003) A Practical synthesis of the platelet fibrinogen antagonist. Elarofiban Org Proc Res Dev 7(6):866–872. https://doi.org/10.1021/op034103o
Deshmukh VV, Mishra AM, Wani DV, Deshpande PK, Bhavsar S, Yeole RD, Patel MV (2014) Sodium salt od (2S,5R)-benzyloxy-7-oxo-1,6-diaza bicyclo [3.2.1] octane-2 carboxylic acid and its preparation. WO 2014/135929 A1
Geddes AM, Klugman KP, Rolinson GN (2007) Introduction: historical perspective and development of amoxicillin/clavulanate. Int J Antimicrob Agents 30(Suppl 2):S109–S112. https://doi.org/10.1016/j.ijantimicag.2007.07.015
Gethers M, Chen I, Abdelraouf K, Nicolau DP (2022) In vivo efficacy of WCK 6777 (Ertapenem/zidebactam) against carbapenemase-producing Klebsiella pneumoniae in the neutropenic murine pneumonia model. J Antimicrob Chemother 77(7):1931–1937. https://doi.org/10.1093/jac/dkac110
Giacobbe DR, Bassetti M, De Rosa FG, Del Bono V, Grossi PA, Menichetti F, Pea F, Rossolini GM, Tumbarello M, Viale P, Viscoli C (2018) ISGRI-SITA (Italian study group on resistant infections of the Società Italiana Terapia Antinfettiva). Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther 16(4):307–320. https://doi.org/10.1080/14787210.2018.1447381
Joshi S, Wankhede K, Jadhav S, Pawar S, Ahirrao V, Bhawsar S, Deshpande P, Yeole R, Patel M (2014) A process for preparation of (2S,5R)-7-oxo-6-sulphooxy-2-[N'-((3R)-piperidin-3-carbonyl)hydrazinocarbonyl]-1,6-diaza-bicyclo[3.2.1]octane, WO2014/135931
Joshi S, Jadhav SB, Rane V, Bhawsar S, Deshpande PK, Yeole RD, Patel MV (2015) A process for preparation of (2S,5R)-6-sulphooxy-7-oxo-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-1,6-diaza-bicyclo[3.2.1] octane. WO 2015/110885 A1
Karlowsky JA, Hackel MA, Bouchillon SK, Sahm DF (2020) In vitro activity of WCK 5222 (Cefepime-Zidebactam) against worldwide collected gram-negative bacilli not susceptible to carbapenems. Antimicrob Agents Chemother 64(12):e01432-e1520. https://doi.org/10.1128/AAC.01432-20
Kong KF, Schneper L, Mathee K (2010) Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS 118(1):1–36. https://doi.org/10.1111/j.1600-0463.2009.02563.x
Lepak AJ, Zhao M, Andes DR (2019) WCK 5222 (Cefepime/Zidebactam) pharmacodynamic target analysis against metallo-β-lactamase producing enterobacteriaceae in the neutropenic mouse pneumonia model. Antimicrob Agents Chemother 63(12):e01648-e1719. https://doi.org/10.1128/AAC.01648-19
Magnus P, Thurston LS (1991) Synthesis of the vinblastine-like antitumor bis-indole alkaloid navelbine analog desethyldihydronavelbine. J Org Chem 56(3):1166–1170. https://doi.org/10.1021/jo00003a045
Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A (2017) WCK 5107 (Zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent β-lactam enhancer activity against pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61(6):e02529-e2616. https://doi.org/10.1128/AAC.02529-16
Mushtaq S, Garello P, Vickers A, Woodford N, Livermore DM (2022a) Activity of ertapenem/zidebactam (WCK 6777) against problem enterobacterales. J Antimicrob Chemother 77(10):2772–2778. https://doi.org/10.1093/jac/dkac280
Mushtaq S, Vickers A, Chaudhry A, Woodford N, Livermore DM (2022b) Inoculum effects of cefepime/zidebactam (WCK 5222) and ertapenem/zidebactam (WCK 6777) for Enterobacterales in relation to β-lactamase type and enhancer effect, as tested by BSAC agar dilution. J Antimicrob Chemother 77(7):1916–1922. https://doi.org/10.1093/jac/dkac108
Noguchi JK, Gill MA (1988) Sulbactam: a beta-lactamase inhibitor. Clin Pharm 7(1):37–51
Papp-Wallace KM (2019) The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections. Expert Opin Pharmacother 20(17):2169–2184. https://doi.org/10.1080/14656566.2019.1660772
Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, Rudin SD, Rather PN, Bhavsar S, Tadiparthi R, Deshpande PK, Patil V, Yeole R, Bhagwat SS, Patel MV, Akker F, Bonomo RA (2018) Strategic approaches to overcome resistance against gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 61(9):4067–4086. https://doi.org/10.1021/acs.jmedchem.8b00091
Patel M, Deshpande P, Bhawsar S, Bhagwat S, Jafri M, Mishra A, Pavase L, Gupta S, Kale R, Joshi S (2013) 1,6-diaza- bicyclo[3.2.1]octane-7-one derivatives and their use in treatment of bacterial infections. WO 2013/030733A1
Patel M, Deshpande P, Bhawsar S, Bhagwat S, Jafri M, Mishra A, Pavase L, Gupta S, Kale R, Joshi S (2014) 1,6-diazabicyclo[3,2,1]octan-7-one derivatives and their use in the treatment of bacterial infections. US 8822450B2
Pawar SS, Jadhav SB, Mishra AC, Rane V, Deshpande PK, Yeole RD, Patel MV (2016) A process for preparation of (2S,5R)-7-oxo-6-sulphooxy-2-[((3R)-pyrrolidine-3-carbonyl)-hydrazinocarbonyl]-1,6-diaza-bicyclo[3.2.1] octane. US 2016/0002234 A1
Pund AP, Rane VP, Raut VT, Ahirrao VK, Yeole RD, Joshi S, Bhavsar S, Rafeeq M, Yadav R, Merwade AY (2023) Synthesis and control of process-related impurities in the β-lactamase inhibitor drug substance Zidebactam. Synth Commun 53(13):1041–1052. https://doi.org/10.1080/00397911.2023.2209677
Rane V, Ahirrao V, Patil K, Jadhav R, Yeole R (2020) Impurity profiling of a novel β-lactam enhancer: zidebactam. Chromatographia 83(3):423–437. https://doi.org/10.1007/s10337-019-03845-3
Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK (2017) WCK 5222 (Cefepime-Zidebactam) antimicrobial activity against clinical isolates of gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61(5):e00072-e00117. https://doi.org/10.1128/AAC.00072-17
Schoonover LL, Occhipinti DJ, Rodvold KA, Danziger LH (1995) Piperacillin/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. Ann Pharmacother 29(5):501–514. https://doi.org/10.1177/106002809502900510
Toussaint KA, Gallagher JC (2015) β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother 49(1):86–98. https://doi.org/10.1177/1060028014556652
Acknowledgements
Authors are thankful to Dr. Vipul Rane and Mr. Badrinarayan Chandak for providing analytical data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhavsar, S., Joshi, S., Deshmukh, V. et al. Design and development of an efficient convergent synthetic strategy for novel β-lactam enhancer zidebactam (WCK 5107). Chem. Pap. 78, 1493–1504 (2024). https://doi.org/10.1007/s11696-023-03176-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03176-6