Abstract
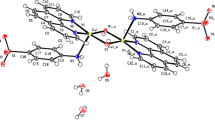
Four complexes [Cu6L6(µ4–O)2(dca)2] (1), [Cu3L3(µ3–OH)Cl2] (2), [Cu3L3(µ3–OH)(OAc)2]·py (3) and [Cu2L4] (4) (HL = phenyl 2-pyridyl ketoxime; dca = dicyanamide anion) have been synthesized and characterized by physicochemical and spectroscopic methods. The structures of all three complexes consist of a single or off-set stacked inverse 9-MC-3 metallacrown formed by three L− ligands and three Cu atoms, and all of the Cu atoms are located in square planar or square pyramidal geometries with different apical ligands. In complex 4, four L− ligands link two Cu atoms to form a dinuclear structure, and the Cu atoms adopt square pyramidal coordination geometry. The in vitro cytotoxicities against four cell lines (A549, HL-60, HT-29 and HCT-116) have been assayed by colorimetric MTT assay. In addition, all four complexes interact strongly with calf thymus DNA, which may be directly responsible for their antitumor activities.











Similar content being viewed by others
References
Becco L, Rodríguez A, Bravo ME, Prieto MJ, Ruiz-Azuara L, Garat B, Moreno V, Gambino D (2012) J Inorg Biochem 109:49–56
Kostova I (2006) Anticancer Agents Med Chem 6:19–32
Ronconi L, Giovagnini L, Marzano C (2005) Inorg Chem 44:1867–1881
Ronconi L, Marzano C, Zanello P (2006) J Med Chem 49:1648–1657
Milacic V, Chen D, Ronconi L, Kristin R, Piwowar L, Fregona D, Dou QP (2006) Cancer Res 66:10478–10486
Soledad BL, Celedonio GR, Isabel GM, Marcos FÁ, Noráh BB (2012) J Inorg Biochem 114:82–93
Fei BL, Li W, Xu WS, Li YG, Long JY, Liu QB, Shao KZ, Su ZM, Sun WY (2013) J Photochem Photobiol B 125:32–41
Kim E, Rye PT, Essigmann JM, Croy RG (2009) J Inorg Biochem 103:256–261
Clarke MJ, Zhu F, Frasca DR (1999) Chem Rev 99:2511–2533
Ott I, Gust R (2007) Arch Pharm 340:117–126
Ronconi L, Sadler PJ (2007) Coord Chem Rev 251:1633–1648
Lorena B, Alejandra R, María EB, María JP, Lena RA, Beatriz G, Virtudes M, Dinorah G (2012) J Inorg Biochem 1009:46–56
Chen QY, Huang J, Guo WJ, Gao J (2009) Spectrochim Acta A 72:648–653
Reddy PR, Shilpa A, Raju N, Raghavaiah P (2011) J Inorg Biochem 105:1603–1612
Jiang M, Li YT, Wu ZY, Liu ZQ, Yan CW (2009) J Inorg Biochem 103:833–844
Marzano C, Pellei M, Tisato F, Santini C (2009) Anticancer Agents Med Chem 9:185–211
Tisato F, Marzano C, Porchia M, Pellei M, Santini C (2010) Med Res Rev 30:708–749
Tardito S, Marchio L (2009) Curr Med Chem 16:1325–1348
Azuara LR, Gómez MEB (2010) Curr Med Chem 17:3606–3615
Li DD, Tian JL, Gu W, Liu X, Zeng HH, Yan SP (2011) J Inorg Biochem 105:894–901
Qiao X, Ma ZY, Xie CZ, Xue F, Zhang YW, Xu JY, Qiang ZY, Lou JS, Chen GJ, Yan SP (2011) J Inorg Biochem 105:728–737
Stamatatos TC, Vlahopoulou JC, Sanakis Y, Raptopoulou CP, Psycharis V, Boudalis AK, Perlepes SP (2004) Inorg Chem Commun 9:814–818
Escuer A, Cordero B, Calvet T (2010) Inorg Chem 49:9752–9754
Afrati T, Pantazaki AA, Raptopoulou C, Terzisb A, Kessissoglou DP (2010) Dalton Trans 39:765–775
Mosmann TJ (1983) Immunol Methods 65:55–63
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR (1988) Cancer Res 48:589–601
Martin D, Abboud KA, Dahnaen KH (1998) Inorg Chem 37:5811–5815
Schraml J (2000) Appl Organomet Chem 14:604–610
Wang F, Yin HD, Yue CH, Cheng S, Hong M (2013) J Organomet Chem 738:35–40
Reichmann ME, Rice SA, Thomas CA, Doty PJ (1954) J Am Chem Soc 76:3047–3053
Satyanarayana S, Dabrowiak JC, Chaires JB (1992) Biochemistry 31:9319–9324
Przyojski JA, Myers NN, Arman HD, Prosvirin A, Dunbar KR, Natarajan M, Krishnan M, Mohan S, Walmsley JA (2013) J Inorg Biochem 127:175–181
Chakraborty A, Gurunatha KL, Muthulakshmi A, Dutta S, Pati SK, Maji TK (2012) Dalton Trans 41:5879–5888
Wang HW, Li DC, Hong M, Dou JM (2013) J Organomet Chem 740:1–9
Afrati T, Zaleski CM, Dendrinou-Samara C, Mezei G, Kampf JW, Pecoraro VL, Kessissoglou DP (2007) Dalton Trans 25:2658–2668
Walz L, Paulus H, Haasem W (1983) Dalton Trans 4:657–664
Dong JF, Li LZ, Xu LLWT, Wang DQ (2011) Chin J Chem 29:259–266
Sahoo BK, Ghosh KS, Bera R, Dasgupta S (2008) Chem Phys 351:163–169
Marmur J (1961) J Mol Biol 3:208–218
Wolfe A, Shimer GH Jr, Meehan T (1987) Biochemistry 26:6392–6396
Baguley BC, Le Bret M (1984) Biochemistry 23:937–943
Selvakumar B, Rajendiran V, Maheswari PV, Stoeckli-Evans H, Palaniandavar M (2006) J Inorg Biochem 100:316–330
Lakowicz JR, Weber G (1973) Biochemistry 12:4161–4170
Raja A, Rajendiran V, Maheswari PU, Balamurugan R, Kilner CA, Halcrow MA, Palaniandavar M (2005) J Inorg Biochem 99:1717–1732
Hegg EL, Burstyn JN (1998) Coord Chem Rev 173:133–165
Acknowledgments
The authors wish to acknowledge financial support from the National Natural Science Foundation of China (Nos. 21271097, 20671048).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, R., Lu, J., Li, D. et al. Syntheses, structures, in vitro cytotoxicities and DNA-binding properties of four copper complexes based on a phenyl 2-pyridyl ketoxime ligand. Transition Met Chem 39, 507–517 (2014). https://doi.org/10.1007/s11243-014-9826-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9826-9