Abstract
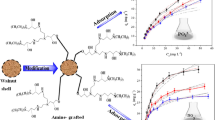
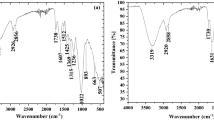
The adsorption of four metals—Pb2+, Zn2+, Cu2+ and Fe3+—in the wastewater from the Clinical Dental Sewage Unit was investigated using the walnut shell (WTS) and its modified forms by activations with acid and base (WTSA and WTSB). The fine fraction of WTS, WTSA and WTSB was characterized by using EDX-RF, FTIR, SEM–EDS and BET. The specific surface area and pore volume were both increased when WTS was activated by acid or base (WTSA or WTSB). The experiment’s optimal pH value was 8, and the adsorption process took place after 300 min. The highest possible adsorption capacities for Pb2+, Zn2+, Cu2+ and Fe3+ by WTSA and WTSB reached (37.89, 33.97, 47.14 and 33.65) mg L−1, and (38.93, 33.02, 43.02, and 32.53) mg L−1. The endothermic and spontaneous nature behavior of HMs adsorption on the adsorbents is confirmed by the positive values of the enthalpy changes (ΔH°) and the negative value of the Gibbs free energy (ΔG°). FTIR and SEM–EDS measurements taken before and after adsorption were compared, and the results showed that there was considerable complexation between the heavy metal ions and the surface functional groups, which were primarily alkyl and alcoholic groups. According to the pseudo-second-order kinetic model and Langmuir isotherm, which are based on physical and chemical prosperity, the second-order chemisorption or chemical action (electron exchange) played a significant role in the adsorption process. WTS, WTSA and WTSB which have been synthesized using inexpensive and environmentally acceptable techniques may be candidates for use as an adsorbent for the treatment of wastewater contaminated with heavy metals.
Graphical abstract






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Babarinde NA, Babalola J, Adebowale R (2006) Biosorption of lead ions from aqueous solution by maize leaf. Int J Phys Sci 1:23–26
Kobya M, Demirbas E, Senturk E, Ince M (2005) Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour Technol 96:1518–1521. https://doi.org/10.1016/j.biortech.2004.12.005
Rahmani K, Mahvi AH, Vaezi F, Mesdaghinia AR, Nabizade R, Nazmara S (2009) Bioremoval of lead by use of waste activated sludge. Int J Environ Res 3:471–476
Romera E, González F, Ballester A et al (2008) Biosorption of heavy metals by Fucus spiralis. Bioresour Technol 99:4684–4693. https://doi.org/10.1016/j.biortech.2007.09.081
Vu HC, Dwivedi AD, Le TT, Seo S (2016) Magnetite graphene oxide encapsulated in alginate beads for enhanced. Chem Eng J. https://doi.org/10.1016/j.cej.2016.08.058
Gautam RK, Sharma SK, Mahiya S, Chattopadhyaya MC (2014) Chapter 1. Contamination of heavy metals in aquatic media: transport, toxicity and technologies for remediation. In: Sharma S (ed) Heavy metals in water: presence, removal and safety. Royal Society of Chemistry, Cambridge, pp 1–24. https://doi.org/10.1039/9781782620174-0000
Chakraborty R, Asthana A, Singh AK et al (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem 102:342–379. https://doi.org/10.1080/03067319.2020.1722811
Sahu JN, Acharya J, Meikap BC (2009) Response surface modeling and optimization of chromium(VI) removal from aqueous solution using Tamarind wood activated carbon in batch process. J Hazard Mater 172:818–825. https://doi.org/10.1016/j.jhazmat.2009.07.075
Jain DSMCK, Yadav AK (2016) Removal of heavy metals from emerging cellulosic low-cost adsorbents: a review. Appl Water Sci. https://doi.org/10.1007/s13201-016-0401-8
Demirbas A (2004) Combustion characteristics of different biomass fuels. Prog Energy Combust Sci 30(219):230. https://doi.org/10.1016/j.pecs.2003.10.004
Kumar PS, Ramalingam S, Sathyaselvabala V (2012) Removal of cadmium (II) from aqueous solution by agricultural waste cashew nut shell. Korean J Chem Eng 29:756–768. https://doi.org/10.1007/s11814-011-0259-2
Ayala J (2020) Removal of zinc, cadmium and nickel from mining waste leachate using walnut shells. Environ Prot Eng. https://doi.org/10.5277/epe190210
Han H, Wang S, Rakita M et al (2018) Effect of ultrasound-assisted extraction of phenolic compounds on the characteristics of walnut shells. Food Nutr Sci 9:1034–1045. https://doi.org/10.4236/fns.2018.98076
Aziz BK, Shwan DMS, Kaufhold S (2020) Characterization of Tagaran natural clay and its efficiency for removal of cadmium (II) from Sulaymaniyah industrial zone sewage. Environ Sci Pollut Res 27:38384–38396. https://doi.org/10.1007/s11356-019-06995-x
Kumar PS, Ramalingam S, Kirupha SD et al (2011) Adsorption behavior of nickel (II) onto cashew nut shell: equilibrium, thermodynamics, kinetics, mechanism and process design. Chem Eng J 167:122–131. https://doi.org/10.1016/j.cej.2010.12.010
Hu X, Jia L, Cheng J, Sun Z (2018) SC research centre for environmental pollution control and resource reuse. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2018.09.003
Li S, Qiu M, Zeng Z, Xue W (2019) Effective modified walnut shell adsorbent: synthesis and adsorption behavior for Pb 2+ and Ni 2+ from aqueous solution. Environ Eng Sci 36:1421–1432. https://doi.org/10.1089/ees.2019.0227
Manohar DM, Noeline BF, Anirudhan TS (2006) Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl Clay Sci 31:194–206. https://doi.org/10.1016/j.clay.2005.08.008
Tulun S, Bahadır T, Şimşek İ, Karataş M (2019) The removal of nickel ions with walnut shell. Turkey Turkish J Eng 3:102–105. https://doi.org/10.31127/tuje.456741
Zafarani HR, Bahrololoom ME, Noubactep C, Tashkhourian J (2015) Green walnut shell as a new material for removal of Cr(VI) ions from aqueous solutions. Desalin Water Treat 55:431–439. https://doi.org/10.1080/19443994.2014.917986
Acknowledgements
This study has not been financed by any government or private funding agencies. We gratefully acknowledge the Razga company for providing the laboratory and instruments for the research, and all who contributed to the conduction of this study, especially Mr. Karokh B. Ali.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: DS and BKM; methodology: DS and BKM; software: DS and BKM; validation: DS and BKM; formal analysis: DS; investigation: DS; resources: DS and BKM; data curation: DS; writing—original draft preparation: DS; writing—review and editing: DS and BKM; visualization: DS and BKM; supervision DS.
Corresponding author
Ethics declarations
Competing interests
Not applicable.
Ethical approval
All of the authors worked on the manuscript, read the final version, and approved it.
Informed consent
All of the authors worked on the manuscript, read the final version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majeed, B.K., Shwan, D.M.S. & Mirjafari, Z. Green and efficient: investigating modified and non-modified walnut shells for heavy metal ion removal in clinical dental sewage treatment. Reac Kinet Mech Cat 136, 2691–2710 (2023). https://doi.org/10.1007/s11144-023-02483-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02483-z