Abstract
Plasma-assisted conversion of ethane (C2H6) can produce value-added chemical building blocks using green electricity. Here we employ a simple packed-bed coaxial dielectric barrier discharge (DBD) reactor to convert C2H6 at mild operating conditions unattainable by conventional thermocatalysis. Ethylene (C2H4), acetylene (C2H2), and methane (CH4) are the main products along with small fractions of C3 and C4 hydrocarbons. Interestingly, the C2H4 selectivity is primarily correlated to C2H6 conversion, dominated by electron dissociation and recombination reactions irrespective of the dielectric properties of the packed bed material (SiO2, Al2O3, ZrO2, TiO2, and BaTiO3), packing material size, supplied power, and C2H6 concentration. While a distortion of the electric field and discharge propagation results in varying dissipated power as materials change, the C2H4 energy yield remains constant. The particle size appears to affect conversion mainly due to pressure alterations. Pd/SiO2 catalyst can change the selectivity, favoring saturated species by expending hydrogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intensive shale gas exploitation has drastically reshaped the energy and chemicals market. Whilst methane (CH4) is its most abundant component, ethane (C2H6) amounts to about 10% and can be transformed into value-added products, such as ethylene (C2H4) and acetylene (C2H2) [1]. The established naphtha cracking for C2H4 (and propylene, C3H6) production has lately been challenged by C2H6 (and propane, C3H8) dehydrogenation due to abundance and low cost of shale gas [2]. The state-of-the-art C2H6 dehydrogenation relies on thermocatalytic conversion requiring high temperatures (i.e., 550 to 700 °C to attain conversion up to 40%) and a considerable energy input, having low energy efficiency in the heat exchanger and producing copious emissions of CO2 [3,4,5]. The difficult activation of the C-H bond, the catalyst deactivation and cost, e.g., Pt-based, or toxicity, e.g., CrOx-based, and the equilibrium limitations are additional challenges of this process. In this context, a highly reactive non-thermal plasma could convert C2H6 at low temperatures with lower energy. Moreover, the operation of a plasma reactor by electrical (including intermittent) energy allows for deployment in remote areas typical of extraction sites [6, 7].
Several plasma reactors have been showcased for non-oxidative coupling [8] and dry reforming [9] of CH4. Dielectric barrier discharges (DBD) are ideal for studying plasma-catalyst interactions, [10, 11] whereas spark discharges promote higher gas temperature and attain higher energy efficiency [12]. Contrariwise, limited literature has been devoted to C2H6, [13,14,15] focusing mainly on oxidative dehydrogenation (ODH) with CO2 as a soft oxidant [16,17,18]. The main products of ODH include C2H4, C2H2, and CH4, whereas C-C coupling reactions into higher hydrocarbon chains have also been observed. Syngas is also produced via this route. In a recent work on ODH, several oxygenated species were produced with selectivity below 4% [18]. All the ODH works report C2H4 yield of about 2-3.5% [17, 18] with the highest value of 14% attained with a Pd catalyst [16]. The only examples of non-oxidative C2H6 dehydrogenation (EDH) include a plasma jet that favors CH4 production over C2H4 [15] and a low-pressure plasma reactor promoting C2H2 and CH4 over C2H4 [13].
Here, we explore plasma-assisted non-oxidative conversion of C2H6 into olefins and light hydrocarbons at atmospheric pressure. A packed-bed coaxial DBD reactor is employed to test packing materials of different dielectric properties and particle size while controlling the dissipated power and residence time. Finally, a silica supported Pd catalyst is compared to bare silica to underscore the catalytic effect on product selectivity and the dielectric effect on C2H6 conversion.
Experimental
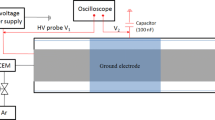
Figure 1 shows a schematic of the experimental set-up, a close-up of the DBD reactor, and a picture with ignited plasma. The coaxial DBD plasma reactor comprises a quartz tube (9.6 mm outer diameter, 6.8 mm inner diameter) around which a copper foil (13 mm long) is wrapped serving as the high voltage electrode. A stainless-steel rod (diameter 3 mm) is placed in the center of the tube and is connected to the circuit ground. The packing bed occupies the tube between the electrodes, while a layer of glass wool (4 μm, Technical Glass Products) is placed before the bed. The feed gas comprises C2H6 (purity > 99.995%, Matheson) and Helium (He, 99.999%, Keen Gas) with flow rates (3–12 sccm for C2H6 and 57–228 sccm for He) regulated via mass flow controllers (GF40 series, Brooks). C2H6 dilution in He is necessary to facilitate plasma ignition and sustainment. In an industrial setting, He could be replaced by Argon to improve plant’s economics. The gas outlet is routed to a gas chromatograph (GC) (Agilent Plot/Q). A sinusoidal high voltage (6–10 kV peak-to-peak) is supplied to the system (PVM500) through the high voltage electrode. A high voltage probe (Tektronix P6015A) and a current monitor (Pearson 2100) are used for monitoring the voltage – current signals via an oscilloscope (Tektronix MDO34). The dissipated power P (W) is calculated from the voltage u (V) and current i (A) waveforms via Eq. (1):
The yield (Y) and product-based selectivity (S) of each product (i) are calculated using Eqs. (2) and (3), respectively:
where \({\nu }_{i}\) is the number of carbon atoms, \({C}_{i}\) is its outlet gas concentration, and \({{C}_{2}{H}_{6}}^{in}\) is the C2H6 inlet concentration.
The packing materials (i.e., SiO2, Al2O3, ZrO2, TiO2, and BaTiO3) were purchased in nanopowder form (US Research Nanomaterials, Inc.), calcined (400 °C for 4 h), pelletized, and sieved (particle size distribution 145–560 μm). The materials filled up the interelectrode zone (details in ESI). The Pd/SiO2 catalyst was prepared by incipient wetness impregnation. A solution of Na2PdCl4 (Sigma Aldrich, > 99.99%) and distilled water was used for impregnation on SiO2. The catalyst was dried overnight at 110 °C before calcination. Catalyst reduction was performed directly in the packed bed through a H2 plasma (10 vol% H2 in He) for 1 h at 10 kV.
(a) Schematic of the DBD packed-bed plasma reactor set-up with auxiliary units: He/C2H6 gas feed, sinusoidal power generator, OES spectrometer, gas chromatograph, electrical waveforms acquisition through oscilloscope, and voltage and current probe. (b) Real image of plasma inside the DBD reactor without packing. (c) Section of the packed reactor with dielectric packing in the plasma region laying on a layer of quartz wool.
Results
Figure 2 shows the effluent concentrations of all detected products for a packed bed comprising 150 mg of SiO2 (particle size 250–425 μm) at an applied voltage of 12 kV, a total flow rate of 60 sccm, with 5% C2H6 in He. The products are mostly CH4, C2H4, and C2H2, and longer isomeric saturated and unsaturated hydrocarbons up to C4 (Fig. 2; the C3 and C4 account for all the saturated and unsaturated species, i.e., 4 species for C3 and 9 species for C4). The concentrations are nearly constant at short times and drop at longer time on stream (i.e., above 90 min) due to a decrease in the conversion likely caused by solid deposit, affecting the plasma. The yellow solid deposit observed mainly on the reactor wall and inner electrode surface and to a lower extent on the particles surface could be ascribed to polymerization of C2 species. A dedicated study presented this phenomenon in plasma-driven polymerization of C2H6 [13]. Hereafter all data reported correspond to 15 min time on stream where the operation is stable and carbon deposit is limited (carbon balance reported in Table S1).
Proposed reaction network of identified species (blue solid line boxes) through main electron impact dissociation and radical recombination reactions (green dashed line boxes). Branching ratios for each dissociation route in purple taken from Ref. [19]
Electron-impact dissociation reactions that form radical species are central in hydrocarbon plasma chemistry [20]. The plasma-induced alkyl and H radicals interact with each other via secondary reactions (neutral-neutral), resulting in a wide range of hydrocarbons with varying carbon number and saturation level. Figure 3 presents the network of electron-impact dissociation reactions leading to the main products reported in Fig. 2. C2H6 dissociation mainly results in C2H4, C2H5, C2H2, and CH4 according to the branching ratio of each dissociation reaction, defined as the contribution of a reaction pathway to the overall cross section of the dissociation processes [19]. C2H4 formation is favored over C2H2 owing to the lower threshold energy of the electron-impact dissociative excitation of C2H6 (i.e., 4 and 6.2 eV for C2H4 and C2H2, respectively) [19]. Nevertheless, C2H2 is also formed via dissociation of C2H4 and C2H3 that in turn forms from C2H4 and C2H5. C2H2 is subject to electron-impact dissociation like any other species. Ethyl radical (C2H5) undergoes electron-impact dissociation to produce chiefly C2H4 and to a less extent C2H2 and CH.
Packing materials of varying dielectric properties are expected to modify the system capacitance and the electric field distribution. A high dielectric constant packing likely promotes partial discharging between solid beads, hindering homogeneous plasma propagation across a packed bed [21,22,23]. Modeling has shown a transition in plasma discharge on the beads’ surface at increasing dielectric constant from a surface ionization discharge to localized filamentary microdischarge [23,24,25]. The coaxial DBD reactor runs with several materials of dielectric constant spanning from 5 to 1000 and without any packing (an empty reactor). The particle size distribution (250–425 μm) and the applied voltage of 10 kV were kept fixed.
The C2H6 conversion and product yields (Fig. 4) for materials of low and medium dielectric constant, such as SiO2 (ε∼5), Al2O3 (ε∼10), and ZrO2 (ε∼25), feature higher C2H6 conversion than the empty reactor and high dielectric constant packings, as TiO2 (ε∼100) and BaTiO3 (ε∼1000). The main products remain as above. The dissipated power for the same applied voltage changes with material. A dielectric medium is seemingly beneficial to conversion and product yield up to a threshold, above which, the plasma diffusion throughout the bed is hindered and is localized as microdischarges that do not favor C2H6 dissociation. Further support was obtained by visual inspection; in contrast to other materials, BaTiO3 features a dimmer plasma inside the bed leading to low emission intensity. However, all materials show filamentary discharge in their electrical waveforms (Figure S2). A comparison at equal dissipated power results in similar waveforms for all materials (Figure S2), but BaTiO3 requires a higher applied voltage to attain the same power, suggesting hindered discharge propagation.
The product selectivity is invariant of the packing material and strongly correlated to the conversion of C2H6 (Fig. 4a), except for non-packed bed and BaTiO3. C2H4 is the main product under our conditions. The selectivity of the other species does not vary substantially with material. However, BaTiO3 promotes low C2H6 conversion which results in enhanced C2H4 selectivity and limited production of recombination products. Conversely, the empty reactor configuration favors generation of C3 and C4 species even at intermediate conversion levels (i.e., ~ 10%). This suggests that microdischarges and surface ionization on the dielectric beads surface promote dehydrogenation over cracking as opposed to gas-phase discharges. While the different dielectric materials have marginal effect on product selectivity, the rising conversion trend attained at decreasing dielectric constant results in the product yield trend of Fig. 4b. SiO2 enables the highest C2H4 yield by allowing more energetic discharges through the bed. Therefore, the C2H4 energy yield (defined as moles of C2H4 per unit of energy dissipated in the plasma) remains constant throughout the experiments as discharge energy is the main driver of C2H4 production. For comparison, our packed bed C2H6 dehydrogenation attains 0.09 molC2H4 kWh− 1, whereas non-oxidative methane coupling in nanosecond pulsed discharge plasma can reach up to 2.2 molC2H4 kWh− 1 [26]. Other promising technologies based on methane coupling, such as pulsed compression and microwave reactors, have shown energy yield of 0.88 molC2H4 kWh− 1 and 0.0012 molC2H4 kWh− 1, respectively [27, 28]. Fig. 4c shows that different dielectric materials affect the plasma discharge and consequently the dissipated power. As a result, C2H6 conversion follows the power trend and in turn drives product yield. By increasing the permittivity of the packing bed, discharge power and conversion drop.
(a) Product selectivity vs. C2H6 conversion for various packings. (b) Product yields and C2H4 energy yield vs. C2H6 conversion. The X-Y axis error bars refer to the variation of conversion and selectivity/yield across 3 repetitions. (c) Product yields, C2H6 conversion, and dissipated power with different dielectric materials in the plasma zone. Shaded curves represent error range. C2H6 feed concentration: 5%. Total flow rate: 60 sccm. Applied voltage: 10 kV (peak-to-peak). A higher C2H6 conversion relates to higher carbon loss due to more carbon deposit (Table S1)
As SiO2 achieves the highest C2H4 yield, we further studied the effect of process parameters. The particle size of SiO2 beads was varied between 145 and 560 μm. The particle size does not play a major role either in the conversion or the product distribution (Fig. 5a) except for a higher conversion attained using smaller particles (i.e., 145–250 μm). The influence of dielectric particles in a plasma environment is not fully discerned; however, modeling studies suggest that the electric field intensifies at the contact point between packing particles, leaving the bed less exposed to an intense electric field [22]. Thus, the smallest beads (i.e., 145–250 μm in Fig. 5a) possess more contact that could enhance localized C2H6 dissociation via increased electric field and electron temperature [29]. Increasing conversion at decreasing particle size has also been observed for CO2 conversion in a DBD plasma reactor [30]. Moreover, the plasma in the packed bed is unstable as a flickering light is seen. This could be due to the pressure increase (from 1.05 to 1.5 atm), resulting from a denser packing that affects the plasma properties. To the contrary, lower residence time is attained with a denser packing, but this phenomenon cannot explain the observed conversion trend.
The gas-bulk temperature is expected to rise at high pressure, but this effect is more relevant in warm plasmas where the H abstraction could dominate the electron-impact dissociation [12]. Nevertheless, the electrical waveforms for different experiments (Figure S3) show the typical filamentary nature of the plasma at all conditions. A slightly lower current observed for the finest particles could possibly be rationalized by the lower reduced electric field at increasing pressure. Therefore, the difference in conversion is more likely caused by the different plasma-surface interactions that result from a denser packing with increasing pressure.
The C2H6 conversion and the product yields for different power levels are displayed in Fig. 5b. The C2H6 conversion depends linearly on the dissipated power (which in turn is directly proportional to the applied voltage), due to increasing electron density and temperature which promote the electron impact dissociation reactions. The C2H4 selectivity decreases upon increasing power, likely due to higher C2H4 electron impact dissociation reactions and radical recombination reactions into larger hydrocarbons (Fig. 3), whereas the selectivity to C2H2 and CH4 remains almost constant.
Increasing the C2H6 inlet molar fraction at a fixed total gas flow rate (60 sccm) hampers both the He breakdown and the plasma sustainment (Fig. 5c) resulting in lower conversion and higher C2H4 selectivity, whereas the ratio CH4/C2H2 and the selectivity of C3 and C4 species are seemingly unaffected.
Since the C2H4 selectivity drops with increasing C2H6 conversion due to extensive electron impact dissociation (Fig. 3), a strategy to enhance the C2H4 productivity would be to lower the contact time by increasing the gas flow rate. Figure 5d displays the production rate (calculated as the product concentration times the total gas flow rate) of the main products and C2H6 conversion vs. gas space velocity (calculated as total gas flow rate over reactor volume). The higher flow rates are characterized by low C2H6 conversions due to the short space times but higher C2H4 production rates. The production rates of CH4 and C2H2 also increase with the total gas flow rate but to a lower degree as they are the products of C2H4 dissociation. Contrariwise, the production rates of C3 and C4 are almost constant.
Despite changing several operating parameters (i.e., packing dielectric constant and particle size, applied power and initial feed concentration), the main products (i.e., CH4, C2H4 and C2H2) are correlated to the conversion. Owing to the reaction network proposed in Fig. 3, C2H4 and C2H2 are mostly generated via electron impact dissociation reactions of C2H6 (Eq. 4), according to Eq. 5 and Eq. 6, respectively. No radical species are included in the mass balance as their concentration is not experimentally measured and should be negligible in the product stream compared to the major stable species due to the high reactivity (low lifetime). Their high reactivity results from high rate coefficients that must be accounted for in a more detailed model. The electron density \({\text{n}}_{e}\) (cm− 3) is unknown but it is constant in all equations. Therefore, electron density is embedded in the reaction rate constants for electron impact dissociation of C2H6, C2H4, and C2H2 that are indicated as \({k}_{1}\),\({k}_{2},\) and \({k}_{3}\) (s− 1), respectively. Molar flow rates, \(F\) of all C2 species are expressed in mol s− 1, whereas species concentrations, \(\text{C}\) are calculated in mol cm− 3 and reactor volume, \(\text{V}\) in cm− 3.
Albeit simplified, this reaction network is consistent with our experimental data. Figure 6a presents all experimental C2H4 and C2H2 production data for the abovementioned parametric studies at varying C2H6 consumption. Both species vary linearly following Eq. 5 and Eq. 6 and the higher slope for the C2H4 production than C2H2 reflects the higher branching ratio of C2H6 dissociation (0.46 vs. 0.11) [19]. These factors are employed to account for the different contributions of the dissociation reaction pathways represented in Fig. 3. Nonetheless, dissociation of C2H4 is favored at higher C2H6 conversion, resulting in increased production of C2H2 (Fig. 6a-b).
Figure 6c reports the reaction rate constants calculated from Eqs. 4–6 based on the experimental dataset of Fig. 4. Both \({k}_{1}\) and \({k}_{2}\) decrease with increasing dielectric constant due to the lower electron density [29] (embedded in the rate constant) attained at lower power (Fig. 4c). The C2H4 dissociation rate constant is one order of magnitude higher than that of C2H6, thus C2H4 is quickly dissociated into C2H2 as confirmed by Fig. 6b. The ratio between the two rate constants eliminates the dependence on the electron density. A rather constant ratio is observed, except for high dielectric media (e.g., BaTiO3) that feature lower dissipated power, hence a lower gas temperature (i.e., 50 °C vs. 75 °C for all other materials). \({k}_{3}\) shows a decreasing trend at increasing dielectric constant as well; however, negative values are obtained indicating that C2H2 dissociation is negligible and radical recombination to form C2H2 (Fig. 3) should not be excluded.
Since the product distribution is driven by the C2H6 conversion rather than the materials or process parameters, a catalytic material was tested to assess if a catalyst could alter the gas-phase chemistry at the solid surface. Palladium (Pd) is an effective hydrogenation catalyst and a few examples have been reported for plasma-generated C2H2 hydrogenation to C2H4 [12, 16]. Therefore, Pd deposited on SiO2 was tested at varying metal loading (i.e., 0.5, 2, 5 wt%). The metal surface is expected to enhance the electric field and charge redistribution near the metal surface [31, 32] and the radical surface quenching compared to an inert surface [33].
Figure 7a confirms that Pd particles on SiO2 seemingly distort the applied electric field similarly to high dielectric constant materials (Fig. 4) as the C2H6 conversion decreases at increasing catalyst content. The charge redistribution is reflected on the electrical waveforms acquired for the metal loading experiments (Figure S2) where more pronounced current spikes are observed upon increasing catalyst loading. Furthermore, the confinement of plasma discharges on the beads contact points could explain the dimmer plasma observed for the high catalyst loading condition.
Besides the electric field effect, Pd/SiO2 modifies the product selectivity (Fig. 7b). The H radicals formed through electron impact dissociation reactions (Fig. 3) are expected to recombine with hydrocarbon radicals on the catalyst surface to generate saturated species. More specifically, C2H2 production is almost completely hindered, and instead more CH4 is attained. Similarly, C2H6 formation via complete hydrogenation of C2H2 alongside recombination of alkyl and hydrogen radicals could be promoted by Pd, contributing to the lower conversion. The C2H4 selectivity is also negatively impacted in favor of CH4 and saturated C3-C4 species. Production of propyne (C3H4) is completely prevented whereas the selectivity of butene (C4H8) on the 5 wt% Pd/SiO2 halves compared to bare SiO2. The observed selectivity shift, where C2H2 production is suppressed even with the lowest catalyst loading, proves the catalytic activity of the Pd particles, thus breaking the correlation between C2H4/C2H2 generation and C2H6 consumption observed for all other experimental sets (Fig. 6). Nonetheless, C2H4 selectivity must be optimized by tuning the degree of hydrogenation. Low catalyst loading could favor partial C2H2 hydrogenation, while low applied power could prevent further dissociation of C2H4. The high activity of the Pd catalyst at low temperature could also enable a post-plasma catalytic step where C2H2 formed in the plasma discharge converts into C2H4 downstream the plasma zone.
Effect of Pd catalyst loading on: (a) C2H6 conversion, product yield, and dissipated power (b) product selectivity and (c) ratio between unsaturated species (i.e., C2H4, C2H2, C3H6, C3H4) and saturated species (i.e., CH4 and C3H8), and H2 outlet concentration. Shaded areas refer to error range. Particle size: 250–425 μm, flow rate: 60 sccm (5% C2H6 in He), applied voltage: 10 kV (peak-to-peak)
A quantification of the hydrogenation effect of the catalyst is possible by comparing all the saturated product species (CH4 and C3H8) with the unsaturated ones (C2H4, C2H2, C3H6, C3H4). The C4 species were not accounted for as their full identification was not possible due to their large number. The effective utilization of the produced H2 by the catalyst to drive hydrogenation, clearly observed in Fig. 7c through the decreasing trend of the ratio between unsaturated and saturated species, mirrors the outlet H2 concentration for the different catalyst loadings.
Furthermore, by promoting hydrogenation, C2H2 oligomerization and ultimately carbon deposit are hindered, as observed for Pd-catalyzed CH4 coupling [11]. Thus, a higher carbon balance is attained at higher catalyst loading (Table S1).
The plasma-assisted activation of C2H6 overcomes thermodynamic limitations as the equilibrium conversion of EDH at the operating temperature (i.e., max 70 °C) is 2*10− 5% vs. the observed one of 20%. The thermocatalytic EDH operates above 600 °C giving C2H4 selectivity > 90% at C2H6 conversion below 50% [5, 34, 35]. The energy demand of these processes compared to the industrial state of the art steam cracking is not currently available. The latter requires 17–21 GJ tC2H4−1 including process energy for separation [36]. The energy demand of the non-optimized plasma reactor is much higher than the industrial benchmark (about 50 times). The ODH plasma processes consume ~ 2.5 and 10X more energy than our reactor, with the exception of the Pd catalyzed ODH that requires about 45% less specific energy input [16,17,18]. Nonetheless, the fully electric process could compete with thermal cracking in terms of carbon footprint. Life Cycle Assessment (LCA) of C2H4 production through plasma-driven CH4 coupling shows that a plasma process powered by renewable wind energy generates CO2 emissions lower than shale gas thermal cracking and comparable to naphtha steam cracking [37].
Conclusions
We explored the non-oxidative upgrading of C2H6 in a coaxial packed-bed DBD plasma reactor to produce light hydrocarbons by varying operational parameters, such as packing material and particle size, dissipated power, C2H6 inlet concentration, and flow rate. The main product is C2H4 followed by C2H2 and then CH4 and C3/C4 hydrocarbons. The chemistry is clearly dominated by electron impact dissociation and radical recombination reactions. Packing materials of varying dielectric properties (i.e., SiO2, Al2O3, ZrO2, TiO2, and BaTiO3) alter the electric field distribution and the dissipated power, hence directly governing C2H6 conversion which drops almost four-fold with increasing dielectric constant. Nonetheless, the C2H4 energy yield remains constant across materials. The most abundant product selectivity (C2H4, C2H2 and CH4) correlates linearly with the C2H6 conversion. The introduction of a Pd catalyst on the SiO2 support distorts the electric field and alters the product selectivity. Hydrogenation produces more saturated species by consuming H2. The H2 concentration is almost 3 times lower over 5 wt% Pd/SiO2 catalyst than bare SiO2.
Data Availability
All data generated during this study is reported in the article and the supplementary material.
References
Porosoff MD, Myint MNZ, Kattel S, Xie Z, Gomez E, Liu P, Chen JG (2015) Identifying different types of catalysts for CO2 reduction by ethane through dry reforming and oxidative dehydrogenation, Angew. Chemie - Int Ed 54:15501–15505. https://doi.org/10.1002/anie.201508128
Kim S, Oh S (2020) Impact of US Shale Gas on the Vertical and Horizontal Dynamics of Ethylene Price. Energies 13. https://doi.org/10.3390/EN13174479
Dai Y, Gao X, Wang Q, Wan X, Zhou C, Yang Y (2021) Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem Soc Rev 50:5590–5630. https://doi.org/10.1039/d0cs01260b
Wang C, Yang B, Gu Q, Han Y, Tian M, Su Y, Pan X, Kang Y, Huang C, Liu H, Liu X, Li L, Wang X (2021) Near 100% ethene selectivity achieved by tailoring dual active sites to isolate dehydrogenation and oxidation. Nat Commun 12:1–8. https://doi.org/10.1038/s41467-021-25782-2
Wu L, Fu Z, Ren Z, Wei J, Gao X, Tan L, Tang Y (2021) Enhanced Catalytic Performance of Fe-containing HZSM-5 for ethane non-oxidative dehydrogenation via Hydrothermal Post-Treatment, ChemCatChem. 13 4019–4028. https://doi.org/10.1002/cctc.202100752
Bogaerts A, Neyts EC (2018) Plasma technology: an Emerging Technology for Energy Storage. ACS Energy Lett 3:1013–1027. https://doi.org/10.1021/acsenergylett.8b00184
Mehta P, Barboun P, Go DB, Hicks JC, Schneider WF (2019) Catalysis enabled by plasma activation of strong Chemical Bonds: a review. ACS Energy Lett 4:1115–1133. https://doi.org/10.1021/acsenergylett.9b00263
Scapinello M, Delikonstantis E, Stefanidis GD (2017) The panorama of plasma-assisted non-oxidative methane reforming. Chem Eng Process Process Intensif 117:120–140. https://doi.org/10.1016/j.cep.2017.03.024
Abiev RS, Sladkovskiy DA, Semikin KV, Murzin DY, Rebrov EV (2020) Non-thermal plasma for process and energy intensification in dry reforming of methane. Catalysts 10:1–41. https://doi.org/10.3390/catal10111358
Kameshima S, Mizukami R, Yamazaki T, Prananto LA, Nozaki T (2018) Interfacial reactions between DBD and porous catalyst in dry methane reforming. J Phys D Appl Phys 51. https://doi.org/10.1088/1361-6463/aaad7d
García-Moncada N, van Rooij G, Cents T, Lefferts L (2021) Catalyst-assisted DBD plasma for coupling of methane: minimizing carbon-deposits by structured reactors. Catal Today 369:210–220. https://doi.org/10.1016/J.CATTOD.2020.04.028
Delikonstantis E, Scapinello M, Van Geenhoven O, Stefanidis GD (2020) Nanosecond pulsed discharge-driven non-oxidative methane coupling in a plate-to-plate electrode configuration plasma reactor. Chem Eng J 380:122477. https://doi.org/10.1016/j.cej.2019.122477
Jensen RJ, Bell AT, Soong DS (1983) Plasma polymerization of ethane. I. Experimental studies of effluent gas composition and polymer deposition rates. Plasma Chem Plasma Process 3:139–161. https://doi.org/10.1007/BF00566018
Jensen RJ, Bell AT, Soong DS (1983) Plasma polymerization of ethane. II. Theoretical analysis of effluent gas composition and polymer deposition rates. Plasma Chem Plasma Process 3:163–192. https://doi.org/10.1007/BF00566019
Sanchez-Gonzalez R, Kim Y, Rosocha LA, Abbate S (2007) Methane and ethane decomposition in an atmospheric-pressure plasma jet. IEEE Trans Plasma Sci 35:1669–1676. https://doi.org/10.1109/TPS.2007.910743
Zhang X, Zhu A, Li X, Gong W (2004) Oxidative dehydrogenation of ethane with CO2 over catalyst under pulse corona plasma. Catal Today 89:97–102. https://doi.org/10.1016/j.cattod.2003.11.015
Trionfetti C, Aǧiral A, Gardeniers HJGE, Lefferts L, Seshan K (2008) Alkane activation at ambient temperatures: unusual selectivities, C-C, C-H bond scission versus C-C bond coupling, ChemPhysChem. 9 533–537. https://doi.org/10.1002/cphc.200700757
Biswas AN, Winter LR, Loenders B, Xie Z, Bogaerts A, Chen JG (2022) Oxygenate production from plasma-activated reaction of CO2and ethane. ACS Energy Lett 7:236–241. https://doi.org/10.1021/acsenergylett.1c02355
Janev RK, Reiter D (2004) Collision processes of C2,3Hy and C 2,3Hy + hydrocarbons with electrons and protons. Phys Plasmas 11:780–829. https://doi.org/10.1063/1.1630794
Heijkers S, Aghaei M, Bogaerts A, Plasma-Based CH (2020) 4 Conversion into Higher Hydrocarbons and H2: modeling to reveal the reaction mechanisms of different plasma sources. J Phys Chem C 124:7016–7030. https://doi.org/10.1021/acs.jpcc.0c00082
Taheraslani M, Gardeniers H (2020) Coupling of CH4 to C2 hydrocarbons in a packed bed DBD plasma reactor: the effect of dielectric constant and porosity of the packing. Energies 13. https://doi.org/10.3390/en13020468
Wang W, Kim HH, Van Laer K, Bogaerts A (2018) Streamer propagation in a packed bed plasma reactor for plasma catalysis applications. Chem Eng J 334:2467–2479. https://doi.org/10.1016/j.cej.2017.11.139
Wang W, Butterworth T, Bogaerts A (2021) Plasma propagation in a single bead DBD reactor at different dielectric constants: insights from fluid modelling. J Phys D Appl Phys 54. https://doi.org/10.1088/1361-6463/abe8ff
Bogaerts A, Zhang QZ, Zhang YR, Van Laer K, Wang W (2019) Burning questions of plasma catalysis: answers by modeling. Catal Today 337:3–14. https://doi.org/10.1016/j.cattod.2019.04.077
Engeling KW, Kruszelnicki J, Kushner MJ, Foster JE (2018) Time-resolved evolution of micro-discharges, surface ionization waves and plasma propagation in a two-dimensional packed bed reactor. Plasma Sources Sci Technol 27. https://doi.org/10.1088/1361-6595/aad2c5
Delikonstantis E, Scapinello M, Stefanidis GD (2018) Low energy cost conversion of methane to ethylene in a hybrid plasma-catalytic reactor system. Fuel Process Technol 176:33–42. https://doi.org/10.1016/j.fuproc.2018.03.011
Slotboom Y, Roosjen S, Kronberg A, Glushenkov M, Kersten SRA (2021) Methane to ethylene by pulsed compression. Chem Eng J 414. https://doi.org/10.1016/j.cej.2021.128821
Julian I, Ramirez H, Hueso JL, Mallada R, Santamaria J (2019) Non-oxidative methane conversion in microwave-assisted structured reactors. Chem Eng J 377. https://doi.org/10.1016/j.cej.2018.08.150
Van Laer K, Bogaerts A (2017) How bead size and dielectric constant affect the plasma behaviour in a packed bed plasma reactor: a modelling study. Plasma Sources Sci Technol 26(8):085007
Uytdenhouwen Y, Van Alphen S, Michielsen I, Meynen V, Cool P, Bogaerts A (2018) A packed-bed DBD micro plasma reactor for CO2 dissociation: does size matter? Chem Eng J 348:557–568
Kruszelnicki J, Engeling KW, Foster JE, Kushner MJ (2021) Interactions between atmospheric pressure plasmas and metallic catalyst particles in packed bed reactors. J Phys D Appl Phys 54. https://doi.org/10.1088/1361-6463/abcc92
Jo S, Kim T, Lee DH, Kang WS, Song YH (2014) Effect of the electric conductivity of a catalyst on methane activation in a dielectric barrier discharge reactor. Plasma Chem Plasma Process 34:175–186. https://doi.org/10.1007/s11090-013-9505-1
Aghalayam P, Bui PA, Vlachos DG (1998) The role of radical wall quenching in flame stability and wall heat flux: hydrogen-air mixtures. Combust Theory Model 2:515–530. https://doi.org/10.1088/1364-7830/2/4/010
Pan Y, Bhowmick A, Wu W, Zhang Y, Diao Y, Zheng A, Zhang C, Xie R, Liu Z, Meng J, Liu D (2021) Titanium Silicalite-1 Nanosheet-Supported platinum for non-oxidative ethane dehydrogenation. ACS Catal 11:9970–9985. https://doi.org/10.1021/acscatal.1c02676
De S, Ould-Chikh S, Aguilar A, Hazemann JL, Zitolo A, Ramirez A, Telalovic S, Gascon J (2021) Stable Cr-MFI catalysts for the nonoxidative dehydrogenation of ethane: Catalytic Performance and Nature of the active Sites. ACS Catal 11:3988–3995. https://doi.org/10.1021/acscatal.0c05170
Ren T, Patel M, Blok K (2006) Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 31:425–451. https://doi.org/10.1016/j.energy.2005.04.001
Delikonstantis E, Igos E, Augustinus M, Benetto E, Stefanidis GD (2020) Life cycle assessment of plasma-assisted ethylene production from rich-in-methane gas streams. Sustain Energy Fuels 4:1351–1362
Acknowledgements
This work was supported from Department of Energy’s Office of Energy Efficient and Renewable Energy’s Advanced Manufacturing Office under Award Number DE-EE0007888-8.3. The Delaware Energy Institute gratefully acknowledges the support and partnership of the State of Delaware toward the RAPID projects.
Funding
This work has received funds from the U.S. Department of Energy, DE-EE0007888-8.3.
Author information
Authors and Affiliations
Contributions
F.C. Conceptualization, Investigation, Writing - Original Draft. P.D. Conceptualization, Writing - Review & Editing. G.D.S. Conceptualization, Writing - Review & Editing. D.G.V. Conceptualization, Writing - Review & Editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cameli, F., Dimitrakellis, P., Stefanidis, G.D. et al. Non-Oxidative Ethane Dehydrogenation in a Packed-Bed DBD Plasma Reactor. Plasma Chem Plasma Process 43, 2065–2078 (2023). https://doi.org/10.1007/s11090-023-10343-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10343-w