Abstract
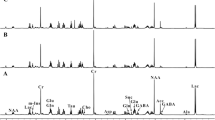
Diabetes mellitus is known to impair glucose metabolism. The fundamental mechanism underlying hyperglycaemia in diabetes mellitus involves decreased utilization of glucose by the brain. However, mechanisms responsible for progressive failure of glycaemic regulation in type I (IDDM) diabetes need extensive and proper understanding. Hence the present study was initiated. Type I diabetes was induced in albino rat models with alloxan monohydrate (40 mg/Kg iv). Cerebral cortex and medulla oblongata were studied 48 h after alloxanisation. Diabetes caused an elevation in glucose, glutamate, aspartate, GABA and taurine levels and a decline in the glutamine synthetase activity. The activities of brain lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH) exhibited significant decrease during diabetes. Ammonia content increased (P < 0.01) as a function of diabetes. Na+-K+ ATPase showed an elevation (P < 0.01) and Ca++-ATPase activity decreased (P < 0.01). Calcium content enhanced (P < 0.05) in the brain of diabetic rats. A General increase in the brain AMP, ADP and ATP was found on inducing diabetes. Impaired cerebral glucose metabolism accounts for the failure of cerebral glucose homeostasis. The impairment in the glycaemic control leads to disturbances in cerebral glutamate content (resulting in calcium overload and excitotoxic injury) and brain energy metabolism as reflected by alterations occurring in adenine nucleotide and the ATPases. The failure in the maintenance of normal energy metabolism during diabetes might affect glucose homeostasis leading to gross cerebral dysfunction during diabetes.



Similar content being viewed by others
References
Siegfried H (1988) Glucose and related brain metabolism in normal aging. Age 11:150–156
Sturt A, Lipton P, Rosenberg A (1994) Excitatory amino acids as a final common pathway of neurologic disorders. Mech dis 330:613–622
Leith E, LaNouve KF, Antonetti DA et al (1998) Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Diabetes 47:815
Biesels GJ, Kapelle AC, Bravenboer B et al (1994) Cerebral functions in diabetes mellitus. (Review). Diabetologia 37(7):643–650
Matschinsky FM, Magnuson MA, Zelent D et al (2006) The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 55:1–12
Lam TK, Gutierrez-Juarez R, Pocai A et al (2005) Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 309:943–947
Wright RA, Nukada H (1994) Nerve microenvironment in diabetic neuropathy. Brain 117:1395–1407
Green DA, Littimer SA (1988) Vascular and metabolic factors in the pathogenesis of experimental diabetic neuropathy in mature rats. Diabetes Metab Rev 4:201–221
Lakhman SS, Sharma P, Kaur G et al (1994) Changes in glucose metabolism from discrete regions of rat brain and its relation ship to reproductive failure during experimental diabetes. Mol Cell Biochem 141:97–102
Ali F, Murthy ASN, Baquer NZ (1980) Lactate dehydrogenase isozymes in diabetic rats. Indian J Exp Biol 17:42–44
Murthy ASN, Baquer NZ (1981) Effect of alloxan diabetes on rat brain pyruvate dehydrogenase. Indian J Biochem Biophys 18(Suppl):52–58
Biessels GJ, van der Heide LP, Kamal A et al (2002) Ageing and diabetes: implications for brain function. Eur J Pharmacol 441(1–2):1–14
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19
Oser BL (ed) (1965) In: Hawk’s physiological chemistry. McGraw-Hill, New York
Giri V, Rao NA (1952) Circular paper chromatography: a technique for the separation and identification of amino acids. J Ind Inst Sci 34:95–105
Colowick SP, Kaplan NO (eds) (1965) In: Methods in enzymology, vol 6. Academic Press, New York, pp 613–614
Rowe B, Ronzio RN, Wellner VP et al (1973) Inhibition of glutamine synthetase by methionine sulfiximine. Anal Biochem 54:304–306
Margulius SK, Seligman AM (1960) A colorimetric method for the estimation of Succinic dehydrogenase activity. J Biol Chem 235(2):499–503
Lowry OH, Rosenbrough NJ, Farr AL et al (1951) Protein measurements with the folin phenol reagent. J Biol Chem 193:265–275
Koch RB (1969) Chlorinated hydrocarbon insecticides. Inhibition of rabbit brain ATPase activities. J Neurochem 16:269–271
Davis JR, Harris RN (1963) Rapid colorimetric determination of adenine compounds. Anal Biochem 5:64–65
Lajtha A (1972) Handbook of neurochemisty, vol VII. Plenum Press, New York
Chol DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Merimee TJ (1990) Diabetic retinopathy. W Eng Med 322(14):978–983
El Idrissi A, Ekkhart T, Jeong Ji E et al (1999) Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 19(21):9459–9468
Albrecht J, Wegrzynowicz M (2005) Endogenous neuro-protectants in ammonia toxicity in the central nervous system: facts and hypotheses. Metab Brain Dis 20(4):253–263
Ahmed N, Zahra N (2008) Brain lactate dehydrogenase activity and heterogeneity during diabetes: Protection by Green tea (Camellia sinensis (L.) O. Kuntze. Phcog Mag 15:170–173
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates anaerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Madhu Char, Carol KL, Lam PYT et al (2008) Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes 57:836–840
Ahmed N (2009) Alloxan–diabetes induced oxidative stress and impairment of oxidative defence system in rat brain: neuroprotective effects of Cichorium Intybus. Int J Diabetes Metab 17:105–109
Wei Y-H, Lu C-Y, Wei C-Y et al (2001) Oxidative stress in human aging and mitochondrial disease−consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol 44(1):1–11
Madhur MG, Chari S (2005) Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Ind J Physiol Pharmacol 49(2):187–192
Rema M, Mohan V, Bhaskar A et al (1995) Does oxidant stress play a role in diabetic neuropathy. Ind J Ophthal 43(1):17–21
Kesavulu MM, Giri R, Rao K et al (2000) Lipid peroxidation and antioxidant enzymes levels in type 2 diabetics with microvascular complications. Diabetes Metab 26(3):387–392
Ahmed N (2008) Proteolytic activity in the brain of alloxan diabetic rats: presence of a proteolytic activator in the cerebral extract. Int J Diabetes Dev Ctries 28(3):83–85. doi:10.4103/0973-3930.44078
Atkinson DE (1968) The energy charge of the adenylate pool as a regulatory parameter. Biochem 7:4030–4034
Acknowledgments
Financial assistance provided to Dr. Nayeemunnisa for the research project by the University Grants Commission, India is gratefully acknowledged. The authors are thankful to Prof. B.G. Shivananda for encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, N., Zahra, N. Neurochemical Correlates of Alloxan Diabetes: Glucose and Related Brain Metabolism in the Rat. Neurochem Res 36, 494–505 (2011). https://doi.org/10.1007/s11064-010-0369-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0369-y