Abstract
A two-step reaction method was used to synthesize a series of rhodanine-based Schiff bases (2–33) that were characterized using spectroscopic techniques. All compounds were assessed for α-amylase inhibitory and radical scavenging (DPPH and ABTS) activities. In comparison to the standard acarbose (IC50 = 9.08 ± 0.07 µM), all compounds demonstrated good to moderate α-amylase inhibitory activity (IC50 = 10.91 ± 0.08–61.89 ± 0.102 µM). Compounds also demonstrated significantly higher DPPH (IC50 = 10.33 ± 0.02–96.65 ± 0.03 µM) and ABTS (IC50 = 12.01 ± 0.12–97.47 ± 0.13 µM) radical scavenging activities than ascorbic acid (DPPH, IC50 = 15.08 ± 0.03 µM; ABTS, IC50 = 16.09 ± 0.17 µM). The limited structure-activity relationship (SAR) suggests that the position and nature of the substituted groups on the phenyl ring have a vital role in varying inhibitory potential. Among the series, compounds with an electron-withdrawing group at the para position showed the highest potency. Kinetic studies revealed that the compounds followed a competitive mode of inhibition. Molecular docking results are found to agree with experimental findings, showing that compounds reside in the active pocket due to the main rhodanine moiety.
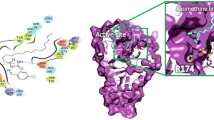
Graphical abstract


















Similar content being viewed by others
References
Arora P, Arora V, Lamba HS, Wadhwa D (2012) Importance of heterocyclic chemistry: a review. IJPSR 3:2947–2954
Kaur M, Singh P (2019) Targeting tyrosine kinase: development of acridone-pyrrole-oxindole hybrids against human breast cancer. Bioorg Med Chem Lett 29:32–35
Badillo JJ, Hanhan NV, Franz AK (2010) Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery. Curr Opin Drug Discov Dev 13:758–776
Singh P, Mothilal S, Kerru N, Singh-Pillay A, Gummidi L, Erukainure OL, Islam MS (2019) Comparative α-glucosidase and α-amylase inhibition studies of rhodanine–pyrazole conjugates and their simple rhodanine analogs. Med Chem Res 28:143–159
Ernst R, Roland NI, Gordon AA (1947) Rhodanine. Org Synth 27:73
Nencki M (1877) On the action of monochloroacetic acid on sulfocyanic acid and its salts. J Pract Chem 16:1–17
Kawakami M, Koya K, Ukai T, Tatsuta N, Ikegawa A, Ogawa K, Shishido T, Chen LB (1998) Structure-activity of novel rhodacyanine dyes as antitumor agents. J Med Chem 41:130–142
Villain-Guillot P, Gualtieri M, Bastide L, Roquet F, Martinez J, Amblard M, Pugniere M, Leonetti JP (2007) Structure-activity-relationships of phenyl-furanyl-rhodanines as inhibitors of RNA polymerase with antibacterial activity on biofilms. J Med Chem 50:4195–4204
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita MG (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2, 4-thiazolidinediones. Bioorg Med Chem 10:1077–1084
Maccari R, Del Corso A, Giglio M, Moschini R, Mura U, Ottanà R (2011) In-vitro evaluation of 5-arylidene-2-thioxo-4-thiazolidinones active as aldose reductase inhibitors. Bioorg Med Chem Lett 21:200–203
Sim MM, Ng SB, Buss AD, Crasta SC, Goh KL, Lee SK (2002) Benzylidene rhodanines as novel inhibitors of UDP-N-acetylmuramate/L-alanine ligase. Bioorg Med Chem Lett 12:697–699
Grant EB, Guiadeen D, Baum EZ, Foleno BD, Jin H, Montenegro DA, Nelson EA, Bush K, Hlasta DJ (2000) The synthesis and SAR of rhodanine as novel class C β-lactamase inhibitors. Bioorg Med Chem Lett 10:2179–2182
Cutshall NS, O’Day C, Prezhdo M (2005) Rhodanine derivatives as inhibitors of JSP-1. Bioorg Med Chem Lett 15:3374–3379
Sing WT, Lee CL, Yeo SL, Lim SP, Sim MM (2001) Aryl alkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Bioorg Med Chem Lett 11:91–94
Xu LL, Zheng CJ, Sun LP, Miao J, Piao HR (2012) Synthesis of novel 1, 3-diaryl pyrazole derivatives bearing rhodanine-3-fatty acid moieties as potential antibacterial agents. Eur J Med Chem 48:174–178
Chauhan K, Sharma M, Singh P, Kumar V, Shukla PK, Siddiqi MI, Chauhan PM (2012) Discovery of a new class of dithiocarbamates and rhodanine scaffolds as potent antifungal agents: synthesis, biology and molecular docking. MedChemComm 3:1104–1110
Murugan R, Anbazhagan S, Narayanan SS (2009) Synthesis and in-vivo antidiabetic activity of novel dispiropyrrolidines through [3+2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives. Eur J Med Chem 44:3272–3279
Li W, Zhai X, Zhong Z, Li G, Pu Y, Gong P (2011) Design, synthesis and evaluation of novel rhodanine-containing sorafenib analogs as potential antitumor agents. Arch der Pharm 344:349–357
Jiang S, Tala SR, Lu H, Abo-Dya NE, Avan I, Gyanda K, Lu L, Katritzky AR, Debnath AK (2011) Design, synthesis, and biological activity of novel 5-((arylfuran/1 H-pyrrol-2-yl) methylene)-2-thioxo-3-(3-(trifluoromethyl) phenyl) thiazolidin-4-ones as HIV-1 fusion inhibitors targeting gp41. J Med Chem 54:572–579
Talele TT, Arora P, Kulkarni SS, Patel MR, Singh S, Chudayeu M, Kaushik-Basu N (2010) Structure-based virtual screening, synthesis, and SAR of novel inhibitors of hepatitis C virus NS5B polymerase. Bioorg Med Chem 18:4630–4638
Irvine MW, Patrick GL, Kewney J, Hastings SF, MacKenzie SJ (2008) Rhodanine derivatives as novel inhibitors of PDE4. Bioorg Med Chem Lett 18:2032–2037
Abu-Dief AM, El-Khatib RM, Aljohani FS, Al-Abdulkarim HA, Alzahrani S, El-Sarrag G, Ismael M (2022) Synthesis, structural elucidation, DFT calculation, biological studies and DNA interaction of some aryl hydrazone Cr3+, Fe3+, and Cu2+ chelates. Comput Biol Chem 97:107643
Abu-Dief AM, El-Khatib RM, Aljohani FS, Alzahrani SO, Mahran A, Khalifa ME, El-Metwaly NM (2021) Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J Mol Struct 1242:130693
Abu-Dief AM, El-Sagher HM, Shehata MR (2019) Fabrication, spectroscopic characterization, calf thymus DNA binding investigation, antioxidant and anticancer activities of some antibiotic azomethine Cu (II), Pd (II), Zn (II) and Cr (III) complexes. Appl Organomet Chem 33(8):4943
Abu-Dief AM, Abdel-Rahman LH, Abdel-Mawgoud AAH (2020) A robust in vitro anticancer, antioxidant, and antimicrobial agents based on new metal-azomethine chelates incorporating Ag (I), Pd (II), and VO (II) cations: Probing the aspects of DNA interaction. Appl Organomet Chem 34(2):5373
Abdel Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM, Adam AM, Ibrahim EMM (2018) Sonochemical synthesis, structural inspection and semiconductor behavior of three new nano-sized Cu (II), Co (II) and Ni (II) chelates based on tri-dentate NOO imine ligand as precursors for metal oxides. Appl Organomet Chem 32(3):4174
Inzucchi SE (2002) Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 287:360–372
Chauhan LK, Chopra J, Vanangamudi M, Tripathi IP, Bhargava A, Goswami AK, Baroliya PK (2022) Hydroxytriazenes incorporating sulphonamide derivatives: evaluation of antidiabetic, antioxidant, anti-inflammatory activities, and computational study. Mol Divers. https://doi.org/10.1007/s11030-022-10420-w
Singh VP, Nidhar M, Yadav P, Kumar R, Sonker P, Tewari AK (2022) Design, synthesis, and molecular modeling of heterodimer and inhibitors of α-amylase as hypoglycemic agents. Mol Divers. https://doi.org/10.1007/s11030-022-10414-8
Rotella DP (2004) Novel “second-generation” approaches for the control of type 2 diabetes. J Med Chem 47:4111–4112
Groop L, Orho-Melander M (2001) The dysmetabolic syndrome. J Inter Med 250:105–120
DeFronzo RA, Bonadonna RC, Ferrannini E (1992) Pathogenesis of NIDDM: a balanced overview. Diabetes Care 15:318–368
Sun H, Wang D, Song X, Zhang Y, Ding W, Peng X, Zhang X, Li Y, Ma Y, Wang R, Yu P (2017) Natural prenylchalconaringenins and prenylnaringenins as antidiabetic agents: α-glucosidase and α-amylase inhibition and in-vivo antihyperglycemic and antihyperlipidemic effects. J Agric Food Chem 65:1574–1581
Aryangat AV, Gerich JE (2010) Type 2 diabetes: postprandial hyperglycemia and increased cardiovascular risk. Vasc Health Risk Manage 6:145–155
Campos C (2012) Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med 124:90–97
Hur SJ, Lim BO, Decker EA, McClements DJ (2011) In vitro human digestion models for food applications. Food Chem 125:1–12
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32:193–203
Miller BR, Nguyen H, Hu CJH, Lin C, Nguyen QT (2014) New and emerging drugs and targets for type 2 diabetes: reviewing the evidence. Am Health Drug Benefits 7:452–463
Raghu C, Arjun HA, Anantharaman P (2019) In-vitro and in-silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr Polym 209:350–355
Hameed S, Seraj F, Rafique R, Chigurupati S, Wadood A, Rehman AU, Venugopal V, Salar U, Taha M, Khan KM (2019) Synthesis of benzotriazoles derivatives and their dual potential as α-amylase and α-glucosidase inhibitors in-vitro: structure-activity-relationship, molecular docking, and kinetic studies. Eur J Med Chem 183:111677
Khan AN, Khan RA, Ahmad M, Mushtaq N (2015) Role of antioxidant in oxidative stress and diabetes mellitus. J Pharmacogn Phytochem 3:217–220
Saltiel AR, Olefsky JM (1996) Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 45:1661–1669
Bayindir S, Yararli K (2019) The easy synthesis of new N-substituted 5-oxindoline-rhodanines and their sensing ability: the recognition of acetate ions in aqueous solution. New J Chem 43:8168–8178
Ivolgina VA, Chernov’yants MS, Popov LD, Suslonov VV, Borodkin GS, Luanguzov NV, Avtushenko NA (2019) Perspective anti-thyroid drug 2-thioxo-5-(3, 4, 5-trimethoxybenzylidene) thiazolidin-4-one: X-ray and thermogravimetric characterization of two novel molecular adducts, obtained by interaction with I2. J Mol Str 1180:629–635
Dumas ME, Chen GY, Kendrick ND, Xu G, Larsen SD, Jana S, Waterson AG, Bauer JA, Hancock W, Sulikowski GA, Ohi R (2019) Dual inhibition of Kif15 by oxindole and quinazolinedione chemical probes. Bioorg Med Chem Lett 29:148–154
Xu JF, Wang TT, Yuan Q, Duan YT, Xu YJ, Lv PC, Wang XM, Yang YS, Zhu HL (2019) Discovery and development of novel rhodanine derivatives targeting enoyl-acyl carrier protein reductase. Bioorg Med Chem 27:1509–1516
Kaminskyy D, Kryshchyshyn A, Lesyk R (2017) Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin Drug Discov 12:1233–1252
Karaman M, Temel Y, Bayindir S (2020) Inhibition effect of rhodanines containing benzene moieties on pentose phosphate pathway enzymes and molecular docking. J Mol Str 1220:128700
Siddiqi KS, Kureshy RI, Khan NH, Khan LA, Tabassum S, Zaidi SAA (1985) Characterization and toxicity of organotin (IV) halide complexes of cyclohexanone spirothiazolidinone and 3-aminorhodanine. Indian J Chem 24:954–956
Petlichnaya LI (1967) Double reactivity of 3-aminorhodanines. Khim Geterotsikl Soedin 4:649–652
Tabatabaee M, Heravi MM, Sharif M, Esfandiyari F (2011) Fast and efficient method for imination of N-aminorhodanine using inorganic solid support under microwave irradiation and classical heating. J Chem 8:535–540
Bayindir S, Caglayan C, Karaman M, Gülcin İ (2019) The green synthesis and molecular docking of novel N-substituted rhodanines as effective inhibitors for carbonic anhydrase and acetylcholinesterase enzymes. Bioorg Chem 90:103096
Mizufune H, Yamamoto H, Nakamura M, Miki S (2008) A new efficient synthetic strategy for N-(dialkylamino) azacycle as a tetrasubstituted hydrazine derivative using sodium triacyloxyborohydride. Tetrahedron 64:6275–6280
Makki MST, Al-Romaizan AN, Abdel-Rahman RM (2010) Synthesis of some more new sulfur-containing heterocyclic compounds as biocidal agents-part II: synthetic of 2-thioxo thiazole/thia diazole and 3-thioxo-1, 3, 4-triazole derivatives. JCCE 4:34–43
Khan M, Alam A, Khan KM, Salar U, Chigurupati S, Wadood A, Ali F, Mohammad JI, Riaz M, Perveen S (2018) Flurbiprofen derivatives as novel α-amylase inhibitors: biology-oriented drug synthesis (BIODS), in-vitro, and in-silico evaluation. Bioorg Chem 81:157–167
Chigurupati S (2020) Antioxidant and antidiabetic properties of Phyllanthus acidus (L.) skeels ethanolic seed extract. Int Food Res J 27:775–782
Taha M, Irshad M, Imran S, Rahim F, Selvaraj M, Almandil NB, Mosaddik A, Chigurupati S, Nawaz F, Ismail NH, Ibrahim M (2019) Thiazole based carbohydrazide derivatives as α-amylase inhibitor and their molecular docking study. Heteroat Chem 7502347:1–8
Yousuf S, Khan KM, Salar U, Chigurupati S, Muhammad MT, Wadood A, Aldubayan M, Vijayan V, Riaz M, Perveen S (2018) 2ʹ-Aryl and 4ʹ-arylidene substituted pyrazolones: as potential α-amylase inhibitors. Eur J Med Chem 159:47–58
Ramírez-Escudero M, Gimeno-Pérez M, González B, Linde D, Merdzo Z, Fernández-Lobato M, Sanz-Aparicio J (2016) Structural analysis of β-fructofuranosidase from Xanthophyllomyces dendrorhous reveals unique features and the crucial role of N-glycosylation in oligomerization and activity. J Biol Chem 291:6843–6857
Salar U, Khan KM, Chigurupati S, Syed S, Vijayabalan S, Wadood A, Riaz M, Ghufran M, Perveen S (2019) New hybrid scaffolds based on hydrazinyl thiazole substituted coumarin; as novel leads of dual potential; in-vitro α-amylase inhibitory and antioxidant (DPPH and ABTS radical scavenging) activities. Med Chem 15:87–101
Salar U, Khan KM, Chigurupati S, Syed S, Vijayabalan S, Wadood A, Riaz M, Ghufran M, Perveen S (2019) New hybrid scaffolds based on hydrazinyl thiazole substituted coumarin; as novel leads of dual potential; in vitro α-amylase inhibitory and antioxidant (DPPH and ABTS radical scavenging) activities. Med Chem 15:87–101
Abu-Dief AM, El-Metwaly NM, Alzahrani SO, Alkhatib F, Abualnaja MM, El-Dabea T, Ali MAEAA (2021) Synthesis and characterization of Fe (III), Pd (II) and Cu (II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J Mol Liq 326:115277
Abu-Dief AM, El-khatib RM, El Sayed SM, Alzahrani S, Alkhatib F, El-Sarrag G, Ismael M (2021) Tailoring, structural elucidation, DFT calculation, DNA interaction and pharmaceutical applications of some aryl hydrazone Mn (II), Cu (II) and Fe (III) complexes. J Mol Str 1244:131017
Aljohani ET, Shehata MR, Abu-Dief AM (2021) Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+, and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. Appl Organomet Chem 35(4):6169
ULC, C, Molecular Operating Environment (MOE), 2013.08. 1010 Sherbrooke St. West, Suite# 910, Montreal, QC, Canada, H3A 2R7, 2018. 10.
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519
Maurus R, Begum A, Kuo HH, Racaza A, Numao S, Andersen C, Tams JW, Vind J, Overall CM, Withers SG, Brayer GD (2005) Structural and mechanistic studies of chloride-induced activation of human pancreatic α-amylase. Protein Sci 14:743–755
Acknowledgements
Dr. Samuel Attah Egu is thankful to The World Academy of Sciences, Trieste, Italy, for awarding a prestigious Fellowship for the year 2019-2020 under the ICCBS-TWAS Post Doc Fellowship program. The authors also thankfully acknowledge the financial support of Sindh Higher Education Commission (SHEC), Pakistan vide letter No. NO.DD/SHEC/1-14/2014, Project Code SHEC/SRSP/Med-3/15/2021-21.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Egu, S.A., Ali, I., Khan, K.M. et al. Syntheses, in vitro, and in silico studies of rhodanine-based schiff bases as potential α-amylase inhibitors and radicals (DPPH and ABTS) scavengers. Mol Divers 27, 767–791 (2023). https://doi.org/10.1007/s11030-022-10454-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10454-0