Abstract
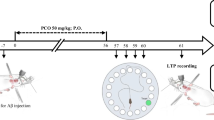
The present study examined the protective effect of sesamin (Ses) on β-amyloid (Aβ)-induced long-term potentiation (LTP) impairment at the PP-DG synapses in male rats. Wistar rats were randomly assigned to seven groups: control, sham, Aβ; ICV Aβ1-42 microinjection, Ses, Aβ + Ses; first, ICV Aβ injections and then receiving Ses, Ses + Aβ: four weeks of pretreatment with Ses and then Aβ injection, and Ses + Aβ + Ses: pre (four weeks) and post (four weeks) treatment with Ses. Ses-treated groups received 30 mg/kg of Ses once a day by oral gavage for four weeks. After the treatment period, the animals were positioned in a stereotaxic device for surgery and field potential recording. The population spike (PS) amplitude and slope of excitatory postsynaptic potentials (EPSP) were evaluated in the DG region. Serum oxidative stress biomarkers (total oxidant status (TOS) and total antioxidant capacity (TAC)) were measured. Aβ impaired LTP induction at the PP-DG synapses evidenced by a decrease in EPSP slope and PS amplitude of LTP. In Aβ rats, Ses increased EPSP slope and PS amplitude of LTP in the DG granular cells. Also, an increase in TOS and a reduction in TAC caused by Aβ were significantly corrected by Ses. Ses could prevent Aβ-induced LTP impairment at the PP-DG synapses in male rats, which can be due to its preventive effects on oxidative stress.







Similar content being viewed by others
Data availability statements
The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Data Availability
Data will be made available on request.
References
Abdul HM, Sultana R, Clair DKS, Markesbery WR, Butterfield DA (2008) Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radical Biol Med 45:1420–1425
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des 16:2766–2778
Ahmadi N, Mirazi N, Komaki A, Safari S, Hosseini A (2021a) Vanillic acid attenuates amyloid β1-40-induced long-term potentiation deficit in male rats: an in vivo investigation. Neurol Res 43:562–569
Ahmadi N, Safari S, Mirazi N, Karimi SA, Komaki A (2021b) Effects of vanillic acid on Aβ1-40-induced oxidative stress and learning and memory deficit in male rats. Brain Res Bull 170:264–273
Alzoubi KH, Mayyas FA, Mahafzah R, Khabour OF (2018) Melatonin prevents memory impairment induced by high-fat diet: role of oxidative stress. Behav Brain Res 336:93–98
Asadbegi M, Yaghmaei P, Salehi I, Komaki A, Ebrahim-Habibi A (2017) Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet-fed rats. Metab Brain Dis 32:827–839
Asadbegi M, Komaki A, Salehi I, Yaghmaei P, Ebrahim-Habibi A, Shahidi S, Sarihi A, Asl SS, Golipoor Z (2018) Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res Bull 137:338–350
Baluchnejadmojarad T, Mansouri M, Ghalami J, Mokhtari Z, Roghani M (2017) Sesamin imparts neuroprotection against intrastriatal 6-hydroxydopamine toxicity by inhibition of astroglial activation, apoptosis, and oxidative stress. Biomed Pharmacother 88:754–761
Bartolotti N, Bennett D, Lazarov O (2016) Reduced pCREB in Alzheimer’s disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Mol Psychiatry 21:1158–1166
Bird CM, Burgess N (2008) The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9:182–194
Bliss TV, Lømo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, Weinshenker D, Levey AI (2013) Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Res Ther 5:1–9
Chen QS, Kagan BL, Hirakura Y, Xie CW (2000) Impairment of hippocampal long-term potentiation by Alzheimer amyloid β-peptides. J Neurosci Res 60:65–72
Criscuolo C, Fabiani C, Bonadonna C, Origlia N, Domenici L (2015) BDNF prevents amyloid-dependent impairment of LTP in the entorhinal cortex by attenuating p38 MAPK phosphorylation. Neurobiol Aging 36:1303–1309
D’Anca M, Fenoglio C, Serpente M, Arosio B, Cesari M, Scarpini EA, Galimberti D (2019) Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front Aging Neurosci 11:232
de San Luis CO, Ryan TJ (2022) Understanding the physical basis of memory: molecular mechanisms of the engram. J Biol Chem 101866
Diniz BS, Teixeira AL (2011) Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. NeuroMol Med 13:217–222
Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT (2009) Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. FASEB J 23:2459–2466
Farbood Y, Ghaderi S, Rashno M, Khoshnam SE, Khorsandi L, Sarkaki A, Rashno M (2019) Sesamin: a promising protective agent against diabetes-associated cognitive decline in rats. Life Sci 230:169–177
Fleming R, Zeisel J, Bennet K (2020) World Alzheimer Report 2020
Hong L, Liangliang C, Yi W, Juncheng H, Qin W, Xiaoxiang Z (2012) Anti-aging effect of sesamin and its mechanism of action. Curr Top Nutraceuticals Res 10:173
Hsieh PF, Hou C-W, Yao P-W, Wu S-P, Peng Y-F, Shen M-L, Lin C-H, Chao Y-Y, Chang M-H, Jeng K-C (2011) Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J Neuroinflammation 8:1–10
Ito N, Saito H, Seki S, Ueda F, Asada T (2018) Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J Alzheimers Dis 62:1767–1775
Jarrard LE (1993) On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol 60:9–26
Jeng K-CG, Hou RC, Wang J-C, Ping L-I (2005) Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-κB. Immunol Lett 97:101–106
Karimi SA, Salehi I, Komaki A, Sarihi A, Zarei M, Shahidi S (2013) Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res 1539:1–6
Karimi SA, Komaki A, Salehi I, Sarihi A, Shahidi S (2015) Role of group II metabotropic glutamate receptors (mGluR2/3) blockade on long-term potentiation in the dentate gyrus region of hippocampus in rats fed with high-fat diet. Neurochem Res 40:811–817
Karimi SA, Salehi I, Shykhi T, Zare S, Komaki A (2019) Effects of exposure to extremely low-frequency electromagnetic fields on spatial and passive avoidance learning and memory, anxiety-like behavior and oxidative stress in male rats. Behav Brain Res 359:630–638
Keowkase R, Shoomarom N, Bunargin W, Sitthithaworn W, Weerapreeyakul N (2018) Sesamin and sesamolin reduce amyloid-β toxicity in a transgenic Caenorhabditis elegans. Biomed Pharmacother 107:656–664
Komaki H, Saadat F, Shahidi S, Sarihi A, Hasanein P, Komaki A (2017) The interactive role of CB1 receptors and L-type calcium channels in hippocampal long-term potentiation in rats. Brain Res Bull 131:168–175
Larson EB, Kukull WA, Katzman RL (1992) Cognitive impairment: dementia and Alzheimer’s disease. Annu Rev Public Health 13:431–449
Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Liu Z, Liu X (2017) Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J 31:1494–1507
Lorenzo A, Yankner BA (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci 91:12243–12247
Malenka RC, Nicoll RA (1999) Long-term potentiation–a decade of progress? Science 285:1870–1874
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biol Med 23:134–147
Nouri E, Karimi SA, Raoufi S, Zarei M (2022) Protective effects of L-carnitine against valproic acid-induced memory impairment and anxiety-like behavior in adult rat. Physiol Behav 113853
Omidi G, Karimi SA, Shahidi S, Faraji N, Komaki A (2020) Coenzyme Q10 supplementation reverses diabetes-related impairments in long-term potentiation induction in hippocampal dentate gyrus granular cells: An in vivo study. Brain Res 1726:146475
Palop JJ, Mucke L (2010) Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 13:812–818
Paxinos G, Watson C (2005a) The rat brain in stereotaxic coordinates. Elsevier Academic Press
Paxinos G, Watson C (2005b) The rat brain in stereotaxic coordinates. Elsevier Academic Press, Burlington, San Diego, London
Puzzo D, Piacentini R, Fa M, Gulisano W, Puma DDL, Staniszewski A, Zhang H, Tropea MR, Cocco S, Palmeri A (2017) LTP and memory impairment caused by extracellular Aβ and Tau oligomers is APP-dependent. Elife 6:e26991
Salehi I, Karamian R, Komaki A, Tahmasebi L, Taheri M, Nazari M, Shahidi S, Sarihi A (2015) Effects of vitamin E on lead-induced impairments in hippocampal synaptic plasticity. Brain Res 1629:270–281
Salehi I, Komaki A, Karimi SA, Sarihi A, Zarei M (2018) Effect of garlic powder on hippocampal long-term potentiation in rats fed high fat diet: an in vivo study. Metab Brain Dis 33:725–731
Samandouras G, Teddy P, Cadoux-Hudson T, Ansorge O (2006) Amyloid in neurosurgical and neurological practice. J Clin Neurosci 13:159–167
Samidurai M, Ramasamy VS, Jo J (2018) β-amyloid inhibits hippocampal LTP through TNFR/IKK/NF-κB pathway. Neurol Res 40:268–276
Saura CA, Valero J (2011) The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Rev Neurosci 22:153–169
Scott-McKean JJ, Roque AL, Surewicz K, Johnson MW, Surewicz WK, Costa A (2018) Pharmacological modulation of three modalities of ca1 hippocampal long-term potentiation in the ts65dn mouse model of down syndrome. Neural Plasticity 2018
Su B, Wang X, Nunomura A, Moreira PI, Lee H-g, Perry G, Smith MA, Zhu X (2008) Oxidative stress signaling in Alzheimer’s disease. Curr Alzheimer Res 5:525–532
Tahmasebi L, Komaki A, Karamian R, Shahidi S, Sarihi A, Komaki H (2016) Interaction between paired-pulse facilitation and long-term potentiation during the stimulation of the cannabinoid and vanilloid systems in the dentate gyrus. Brain Res 1643:27–34
Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120:545–555
Taube JS, Schwartzkroin PA (1988) Mechanisms of long-term potentiation: a current-source density analysis. J Neurosci 8:1645–1655
Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno A (2012) Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. SAGE Publications Sage, London
Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M (2002) Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci 99:13217–13221
Wang S-Q, Li D, Yuan Y (2019) Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-β1/Smad pathway. J Physiol Sci 69:861–873
Wilson RS, Leurgans SE, Boyle PA, Bennett DA (2011) Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol 68:351–356
Xia F, Yiu A, Stone SS, Oh S, Lozano AM, Josselyn SA, Frankland PW (2017) Entorhinal cortical deep brain stimulation rescues memory deficits in both young and old mice genetically engineered to model Alzheimer’s disease. Neuropsychopharmacology 42:2493–2503
Zhang L, Zhang H-Q, Liang X-Y, Zhang H-F, Zhang T, Liu F-E (2013) Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behav Brain Res 256:72–81
Zhao TT, Shin KS, Park HJ, Kim KS, Lee KE, Cho YJ, Lee MK (2016) Effects of (-)-sesamin on chronic stress-induced memory deficits in mice. Neurosci Lett 634:114–118
Zhao Y, Wang Q, Jia M, Fu S, Pan J, Chu C, Liu X, Liu X, Liu Z (2019) (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J Nutr Biochem 64:61–71
Zuo Y, Peng C, Liang Y, Ma KY, Chan HYE, Huang Y, Chen Z-Y (2013) Sesamin extends the mean lifespan of fruit flies. Biogerontology 14:107–119
Acknowledgements
The authors are grateful to the staff of the Neurophysiology Research Center, Hamadan University of Medical Sciences for supporting this study.
Funding
This research was carried out at the Hamadan University of Medical Sciences (Grant No.: 140010218738).
Author information
Authors and Affiliations
Contributions
Each author has made substantial intellectual contributions to the design, implementation, and interpretation of studies described. A.A. conducted experiments, data acquisition and drafting the manuscript; S.A.K. and I.S. were responsible for administrative, technical and material support and critical revision of the manuscript for important intellectual content; R.H. contributed technical support and manuscript drafting. A.K. contributed to the study design and supervision, statistical analysis of data, writing and critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal care processes were approved by the Veterinary Ethics Committee of the Hamadan University of Medical Science (Ethic code: IR.UMSHA.REC.1400.456) and were accomplished consistent with the Guidelines of the National Institutes of Health (NIH Publication 80–23, 1996).
Consent for publication
All the authors have approved the manuscript and agree with submission to your esteemed journal.
Consent to participate
Because this research has been done on animal models, “Consent to Participate” not relevant.
Competing interests
The authors confirm that there is no conflict of interest associated with this publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arabi, A., Karimi, S.A., Salehi, I. et al. Effects of sesamin on Aβ1-42-induced oxidative stress and LTP impairment in a rat model of Alzheimer's disease. Metab Brain Dis 38, 1503–1511 (2023). https://doi.org/10.1007/s11011-023-01191-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01191-w