Abstract
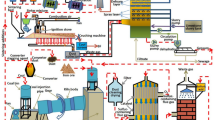
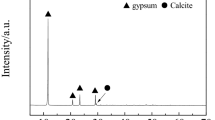
Flue gas desulfurization (FGD) gypsum mainly contains gypsum, which comes from the FGD by using lime or limestone. The effects of calcining temperature and time on calcium sulfide preparation were explored. The gypsum completely decomposed into calcium sulfide by calcining at 900 °C for 30 min. The effect of time on CaS and CO2 reaction were also explored in aqueous solution. The main phase was calcite phase after reaction 6 h, with nearly cubic particles and 1–2 µm in diameter, which calcite can be reused in FGD process. Hydrogen sulfide was produced when CaS reacted with CO2. And then, irregular particle and pellets of elemental sulfur, with 0.5–5 μm in diameter, were obtained by the H2S oxidation in Fe(III) chelate solution.










Similar content being viewed by others
References
Myka A, Łyszczek R, Zdunek A, Rusek P. Thermal analysis of materials based on calcium sulphate derived from various sources. J Therm Anal Calorim. 2022;147:9923–34. https://doi.org/10.1007/s10973-022-11319-2.
Ma X, Tan H, He X. Preparation and surface modification of anhydrous calcium sulfate whiskers from FGD gypsum in autoclave-free hydrothermal system. Energ Source Part A. 2018;40(17):2055–62. https://doi.org/10.1080/15567036.2018.1487481.
Li J, Cao J, Ren Q, Ding Y, Zhu H, Xiong C, et al. Effect of nano-silica and silicone oil paraffin emulsion composite waterproofing agent on the water resistance of flue gas desulfurization gypsum. Constr Build Mater. 2021;287:123055. https://doi.org/10.1080/15567036.2018.1487481.
Zhang K, Wang C, Wang P, Zhang K. Effect of Fe3+ concentration and pH on arsenic removal, migration and speciation transformation in lab-scale wet flue gas desulfurization system. Fuel. 2021;295:120633. https://doi.org/10.1016/j.fuel.2021.120633.
Chang L, Zhao Y, Zhang Y, Yu X, Li Z, Gong B, et al. Mercury species and potential leaching in sludge from coal-fired power plants. J Hazard Mater. 2021;403:123927. https://doi.org/10.1016/j.jhazmat.2020.123927.
Li C, Zong H, Wang S, Xue J, Wu F, Zhang Z, et al. Manganese extraction by reduction−acid leaching from low-grade manganese oxide ores using CaS as reductant. T Nonferr Metal Soc. 2015;25:1677–84. https://doi.org/10.1016/s1003-6326(15)63772-4.
Li Y, Xiao C, Hui R. Calcium sulfide (CaS), a donor of hydrogen sulfide (H2S): A new antihypertensive drug? Med Hypotheses. 2009;73:445–7. https://doi.org/10.1016/j.mehy.2009.03.030.
Eng S, Motekaitis R, Martell A. Degradation of coordinated b-diketonates as iron chelate catalysts during the oxidation of H2S to S8 by molecular oxygen. Inorg Chim Acta. 2000;299:9–15. https://doi.org/10.1016/s0020-1693(99)00434-X.
Ma X, Tan H, Dong F, Li B, Wang J, He X, Liu C. Influence of troilite on the decomposition of ammonium jarosite and estimated activation energy. J Therm Anal Calorim. 2020;139(2):933–9. https://doi.org/10.1007/s10973-019-08480-6.
Piché S, Larachi F. Dynamics of pH on the oxidation of HS- with iron(III) chelates in anoxic conditions. Chem Eng Sci. 2006;61:7673–83. https://doi.org/10.1016/j.ces.2006.09.004.
Yang J, Zhu B, Ma L, Liu H. Investigation of Al2O3 and Fe2O3 transmission and transformation during the decomposition of phosphogypsum. Chin J Chem Eng. 2019;27:1125–31. https://doi.org/10.1016/j.cjche.2018.09.023.
Di Z, Yang F, Cao Y, Zhang K, Guo Y, Gao S, Cheng F. The transformation pathways on the catalytic and stability-promoted CaSO4 reduction in CLC process using Fe2O3 supported. Fuel. 2019;253:327–38. https://doi.org/10.1016/j.fuel.2019.04.141.
Yan Z, Wang Z, Liu H, Tu Y, Yang W, Zeng H, et al. Decomposition and solid reactions of calcium sulfate doped with SiO2, Fe2O3 and Al2O3. J Anal Appl Pyrol. 2015;113:491–8. https://doi.org/10.1016/j.jaap.2015.03.019.
Touzo B, Scrivener K, Glasser F. Phase compositions and equilibria in the CaO-Al2O3- Fe2O3-SO3 system, for assemblages containing ye’elimite and ferrite Ca2(Al, Fe)O5. Cement Concrete Res. 2013;54:77–86. https://doi.org/10.1016/j.cemconres.2013.08.005.
Mihara N, Kuchar D, Kojima Y, Matsuda H. Reductive decomposition of waste gypsum with SiO2, Al2O3, and Fe2O3 additives. J Mater Cycles Waste. 2007;9:21–6. https://doi.org/10.1007/s10163-006-0167-4.
Zhang Z, Wang Y, Zhu L, Li J, Wang F, Yu G. Performance of Fe2O3/Al2O3 oxygen carrier modified by CaCO3 and CaSO4 in chemical looping combustion. Appl Therm Eng. 2019;160(11):3813–113823. https://doi.org/10.1016/j.applthermaleng.2019.113813.
Wei R, Lv X, Yang M, Xu J. Effect of ultrasonic vibration treatment on solid-state reactions between Fe2O3 and CaO. Ultrason Sonochem. 2017;38:281–8. https://doi.org/10.1016/j.ultsonch.2017.03.023.
Acknowledgements
This work was supported by the Research Fund of the Sichuan Science and Technology Program of China (2020YFS0334), Natural Science Foundation of Southwest University of Science and Technology (19zx7130) and State Key Laboratory of Solid Waste Reuse for Building Materials (SWR-2021-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, H., Ye, M., Su, X. et al. Elemental sulfur recovery from FGD gypsum and calcium cyclic utilization in coal-fired power plants. J Therm Anal Calorim 147, 14115–14121 (2022). https://doi.org/10.1007/s10973-022-11724-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11724-7