Abstract
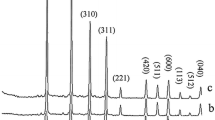
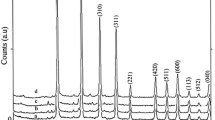
Interaction in the system of lithium carbonate–niobium pentoxide during mechanochemical treatment in air and water has been studied. Prepared samples have been investigated with the help of DTA–TG, XRD, FTIR, Raman, and UV–Vis spectroscopy, adsorption–desorption of nitrogen and TEM. Activation of reagents and direct mechanochemical synthesis are observed at 600–850 and 1,000 rpm, respectively. Lithium metaniobate samples prepared via milling possess high dispersity, defective structure, and improved photocatalytic activity, including under visible illumination.







Similar content being viewed by others
References
Niederberger M, Pinna N, Polleux J, Antonietti M. General soft-chemistry route to perovskites and related materials: synthesis of BaTiO3, BaZrO3, and LiNbO3 nanoparticles. Angew Chem Int Ed. 2004;43:2270–3.
Volk T, Wohlecke M. Lithium niobate defects, photorefraction and photoelectric switching. Berlin: Springer; 2008.
Wu W, Wang K, Li Y, Wu X, Liao S, Wang Q. Nanocrystalline LiMn2O4 preparation and kinetics of thermal process of precursor. J Therm Anal Calorim. 2013;112:1391–9.
Swaan HM, Li Y, Seshan K, et al. The oxidative coupling of methane and the oxidative dehydrogenation of ethane over a niobium promoted lithium doped magnesium oxide catalyst. Catal Today. 1993;16:537–46.
Inoue Y, Watanabe Y. Use of LiNbO3 for design of device-type catalysts with activity controllable functions. Catal Today. 1993;16:487–94.
Xiaoyan L, Kenji K, Kazuya T, et al. Photocatalytic nanoparticle deposition on LiNbO3 nanodomain patterns via photovoltaic effect. Appl Phys Lett. 2007;91:044101–3.
Giocordi JL, Rohrer GS. Spatially selective photochemical reduction of silver on the surface of ferroelectric barium titanate. Chem Mater. 2001;13:241–5.
Zielinska B, Borowiak-Palen E, Kalenzuk RJ. Preparation and characterization of lithium niobate as a novel photocatalyst in hydrogen generation. J Phys Chem Solids. 2008;69:236–42.
Godinho MJ, Ribeiro C, Gonçalves RF, Longo E, Leite ER. High-density nanoparticle ceramic bodies. J Therm Anal Calorim. 2013;111:1351–5.
Su TT, Jiang H, Gong H, Zhai Y. Preparation of nanocrystalline lithium niobate powders at low temperature. Cryst Res Technol. 2010;45:977–82.
Liu M, Xue D, Luo C. Facile synthesis of lithium niobate squares by a combustion route. J Am Ceram Soc. 2006;89:1551–6.
Liu M, Xue D. An efficient approach for the direct synthesis of lithium niobate powders. Solid State Ion. 2006;177:275–80.
Bhagavannarayana G, Ananthamurthy RV, Budakoti GC, et al. A study of the effect of annealing on Fe-doped LiNbO3 by HRXRD, XRT and FT-IR. J Appl Cryst. 2005;38:768–71.
Indris S, Bork D, Heitjans P. Nanocrystalline oxide ceramics prepared by high-energy ball milling. J Mater Synth Process. 2000;8:245–50.
Stojanovic BD. Mechanochemical synthesis of ceramic powders with perovskite structure. J Mater Process Technol. 2003;143–144:78–81.
Rojac T, Kosec M, Malic B, Holc J. The application of a milling map in the mechanochemical synthesis of ceramic oxides. J Eur Ceram Soc. 2006;26:3711–6.
Rojac T, Kosec M, Malic B, Holc J. The mechanochemical synthesis of NaNbO3 using different ball-impact energies. J Am Ceram Soc. 2008;91:1559–65.
Khalameida S, Sydorchuk V, Skubiszewska-Zięba J, et al. Synthesis, thermo-analytical, and spectroscopical studies of dispersed barium titanate. J Therm Anal Calorim. 2010;101:779–84.
Rojac T, Bencan A, Ursic H. Synthesis of a Li- and Ta-modified (K, Na)NbO3 solid solution by mechanochemical activation. J Am Ceram Soc. 2008;91:3789–91.
Timoshevskii AN, Ktalkherman MG, Emel’kin VA, Pozdnyakov BA, Zamyatin AP. High-temperature decomposition of lithium carbonate at atmospheric pressure. High Temp. 2008;46:414–21.
Zeng HC, Tung SK. Synthesis of lithium niobate gels using a metal alkoxide–metal nitrate precursor. Chem Mater. 1996;8:2667–72.
Santulli AC, Zhou H, Berweger S, et al. Synthesis of single-crystalline one-dimensional LiNbO3 nanowires. CrystEngComm. 2010;12:2675–8.
Zhao JP, Liu XR, Qiang LS. Characteristics of the precursors and their thermal decomposition during the preparation of LiNbO3 thin films by the Pechini method. Thin Solid Films. 2006;515:1455–60.
Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JM. Recommendations for the characterization of porous solids. Technical Report. Pure Appl Chem. 1994;66:1739–58.
Dolci F, Di CM, Baricco M, Giamello E. Niobium pentoxide as promoter in the mixed MgH2/Nb2O5 system for hydrogen storage: a multitechnique investigation of the H2 uptake. J Mater Sci. 2007;42:7180–5.
Lemercier T, Quarton M, Fontaine MF, Hague CF. Structural and chemical transformations induced by laser impact on TiO2 and Nb2O5. J Phys Chem Solids. 1997;58:679–84.
Redfield D, Burke WJ. Optical absorption edge of LiNbO3. J Appl Phys (USA). 1974;45:4566–71.
Chao L, Dongfeng X. Mild, quasireverse emulsion route to submicrometer lithium niobate hollow spheres. Langmuir. 2006;22:9914–8.
Thierfelder C, Sanna S, Schindlmayr A, Schmidt WG. Do we know the band gap of lithium niobate? Phys Status Solidi C. 2010;7:362–5.
Zhang W, Sun X, Chen B. Photocatalytic degradation of methyl orange on iron niobate prepared by solid-state reaction. Adv Mater Res. 2010;113–114:2021–4.
Kim TH, Yu YM. Effect of lithium compensation on UV–Vis and IR absorption spectra in LiNbO3 crystals. J Korean Phys Soc. 2002;41:390–4.
Lo YH, Gopal NO, Ke SC. Origin of photoactivity of oxygen-deficient TiO2 under visible light. Appl Phys Lett. 2009;95:083126–8.
Liu G, Yang YG, Wang X, et al. Enhanced photoactivity of oxygen-deficient anatase TiO2 sheets with dominant 001 facets. J Phys Chem C. 2009;113:21784–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalameida, S., Sydorchuk, V., Leboda, R. et al. Preparation of nano-dispersed lithium niobate by mechanochemical route. J Therm Anal Calorim 115, 579–586 (2014). https://doi.org/10.1007/s10973-013-3343-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3343-5