Abstract
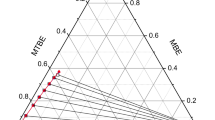
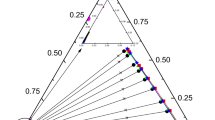
Methyl acrylate has a wide range of uses, but the production process usually produces aqueous by-products. In this study, four commonly used alcohols (1-pentanol, 1-hexanol, 1-heptanol, and 1-octanol) were selected as extractants to explore the liquid–liquid extraction behavior of water + methyl acrylate + extractants. Through the analysis of partition coefficient (D) and separation coefficient (S), it is found that the data of all systems are greater than 1, indicating that all four alkyl alcohols can well separate methyl acrylate from aqueous solution, and 1-octanol is the best. In addition, the consistency between the binary interaction parameters of regression and tie-line data was tested using Matlab tools, proving that the obtained parameters have good consistency.





Similar content being viewed by others
References
Lin, J.W., Zaki, A.H., Wu, H.T., Lin, H.M., Lee, M.J.: Kinetics study on esterification of acrylic acid and ethanol over acidic cation-exchange resin beads amberlyst 35. J. Taiwan Inst. Chem. E. 102, 44–50 (2019)
Han, M., Xu, M.Y., Pang, Y.F., Cao, J.W., Zhang, A.L., Wang, Y.X., Wei, J.J., Song, Y.J., Li, Q.S.: Liquid-liquid equilibrium of water, ethyl acrylate, and alkyl alcohol solvents: extractant screening and thermodynamic analysis. J. Chem. Thermodyn. 177, 106930 (2023)
Xu, X.B., Lin, J.P., Cen, P.L.: Advances in the research and development of acrylic acid production from biomass. Chinese J. Chem. Eng. 14, 419–427 (2006)
Zhao, H., Li, J., Wang, L., Li, C.S., Li, P.: Thermodynamic investigation of 1,3,5-trioxane, methyl acrylate, methyl acetate, and water mixtures, in terms of NRTL and UNIQUAC models. Ind. Eng. Chem. Res. 58, 18378–18386 (2019)
Zhang, H.X.: Liquid-liquid phase equilibria for quinary, quaternary, and ternary systems {water + furfural + acetic acid + cyclopentyl methyl ether + CaCl2}: measurement, effect of salt, and comparative Study. J. Chem. Eng. Data 63, 3338–3344 (2018)
Melissa, C.B., Thiago, C.A., Talita, R.G., Leonardo, H.O., Martin, A.: (Liquid + liquid) equilibria in water + acrylic acid + (1-butanol, or 2-butanol, or 1-pentanol) systems at T = 293.2 K, T = 303.2 K, and T = 313.2 K and atmospheric pressure. J. Chem. Thermodyn. 43, 1381–1388 (2011)
Chen, C.C., Ardila, H.T., Lin, H.M., Lee, M.J.: Liquid-liquid equilibria of ternary aqueous mixtures containing acrylates, acrylic acid, or alkanols. Fluid Phase Equilib. 513, 112555 (2020)
Zhang, L., Yu, Y., Liao, Z., Xu, X., Huang, X., Jia, B., Li, Q.S.: Liquid-liquid equilibrium for the ternary systems water + 1-butanol + 1-hexanol or 1-octanol at 303.15, 31315, and 323.15 K. J. Chem. Eng. Data. 65, 477–486 (2020)
Jcgm, J.C.: Evaluation of measurement data-Guide to the expression of uncertainty in measurement. International Organization for Standardization Geneva ISBN. 50, 134 (2008)
Konieczka, P., Namies’nik, J.: Estimating uncertainty in analytical procedures based on chromatographic techniques. J. Chromatogr. A. 1217, 882–891 (2010)
Barnes, N., Gramajo, M., Sólimo, H.N.: Aqueous phase diagrams containing oxalic acid at 303.15 K. Fluid Phase Equilib. 134, 201–211 (1997)
Butler, J.A.V., Thomson, D.W., MacLennan, W.H.: The free energy of the normal aliphatic alcohols in an aqueous solution. Part I. The partial vapour pressures of aqueous solutions of methyl, n-propyl, and n-butyl alcohols. Part II. The solubilities of some normal aliphatic alcohols in water. Part III. The theory of binary solutions, and its application to aqueous-alcoholic solutions. J. Chem. Soc. 173, 674–686 (1933)
Barnes, N., Gramajo, M., Sólimo, H.N.: Aqueous phase diagrams containing t-aconitic acid + (1-pentanol or + isobutyl acetate or + methyl isobutyl ketone) at 303.15 K. Fluid Phase Equilib. 168, 217–227 (2000)
Duarte, M.M., Lozar, J., Malmary, G., Molinier, J.: Equilibrium diagrams at 19 °C of water-malic acid-2-methyl-1-propanol, water-malic acid-1-pentanol, and water-malic acid-3-methyl-1-butanol ternary systems. J. Chem. Eng. Data 34, 43–45 (1989)
Esquível, M.M., Bernardo-Gil, M.G.: Liquid-liquid equilibria for the systems: water/1-pentanol/acetic acid and water/1-hexanol/acetic acid. Fluid Phase Equilib. 62, 97–107 (1991)
Góral, M.: Cubic equation of state for calculation of phase equilibria in association systems. Fluid Phase Equilib. 118, 27–59 (1996)
Tokunaga, S., Manabe, M., Koda, M.: The solubility of water in 1-alkanols and temperature dependence. Niihama Kogyo Koto Senmon Gakko Kiyo, Rikogaku-Hen. 16, 96–101 (1980)
Herz, W.: About the solubility of some liquids which are difficult to mix with water. Berichte Der Dtsch. Chem. Gesellschaft. 31, 2669–2672 (1898)
Chandy, A., Raja Rao, M.: Ternary liquid equilibria: 1-hexanol-water-fatty acids. J. Chem. Eng. Data 7, 473–475 (1962)
Sazonov, V., Markuzin, N.P., Filippov, V.V.: Liquid-liquid equilibrium in system nitromethane-hexyl alcohol-water. J. Appl. Chem. USSR 49, 817–820 (1976)
Fühner, H.: The water solubility in homologous series. Berichte Der Dtsch. Chem. Gesellschaft (A B Ser.) 57, 510–515 (1924)
Venkataratnam, R.C., Rao, R.J.: Ternary liquid equilibria: acetone-water-nbutanol and acetone-water-n-hexanol system. J. Sci. Ind. Res. B. 17, 108 (1958)
Ratouis, D.M.: Thermodynamic activities of aliphatic alcohols in water and in ringer’s liquid: I aliphatic alcohols slightly soluble in water. Bull. Soc. Chim. Fr. 15, 3318–3322 (1965)
Darwish, N.A., Abdulkarim, M.A., Ashour, I., Dwaidar, A.M., Athamneh, F.S.: Liquid-liquid equilibrium for the system water + acetic acid + 1-heptanol at 2780.1, 293.1, 303.1 and 313.1 K. Fluid Phase Equilib. 200, 277–285 (2002)
Erichsen, L.: The critical solution temperatures in the homologous series of primary normal alcohols. Brennst.-Chem. 33, 166–172 (1952)
Stephenson, R., Stuart, J., Tabak, M.: Mutual solubility of water and aliphatic alcohols. J. Chem. Eng. Data 29, 287–290 (1984)
Vochten, R., Petre, G.: Study of the heat of reversible adsorption at the air-solution interface. I.I Experimental determination of the heat of reversible adsorption of some alcohols. J. Colloid Interface Sci. 42, 320–327 (1973)
Rao, R.C., Rao, M.V.: Ternary Liquid Equilibria: acetone-water-nheptanol & acetone-water-n-octanol Systems. J. Sci. Industr. Res. 20, 283–286 (1961)
Sobotka, G.D.: Lipolytic enzymes: I. studies on the mechanism of lipolytic enzyme actions. J. Biol. Chem. 105, 199–219 (1934)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess gibbs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Harvianto, G.R., Kim, S.E., Jin, I.B., Kang, K.J., Lee, M.: Liquid-liquid equilibria data for the quaternary system of acetic acid, water, p-xylene, and ethyl acetate at 313.15 K and 101.325 kPa. J. Chem. Eng. Data. 61, 780–787 (2016)
Marcilla, A., Reyes-Labarta, J.A., Olaya, M.M.: Fluid phase equilibria should we trust all the published LLE correlation parameters in phase equilibria? Necessity of their assessment prior to publication. Fluid Phase Equilib. 433, 243–252 (2017)
Marcilla, A., Labarta, J.A., Serrano, C., María, D., Olaya, M.: GE models and algorithms for condensed phase equilibrium data regression in ternary systems: limitations and proposals. Open Thermodyn. J. 5, 48–62 (2011)
Author information
Authors and Affiliations
Contributions
QL: Supervision. SJ: Conceptualization and Methodology. HY: Data curation and Writing, Original draft preparation. WF: Investigation and analysis.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Jiang, S., Yan, H. et al. Liquid–Liquid Equilibrium for the Ternary System of Water, Methyl Acrylate, and Different Solvents at 303.2 K under 101.3 kPa. J Solution Chem (2024). https://doi.org/10.1007/s10953-023-01349-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10953-023-01349-1