Abstract
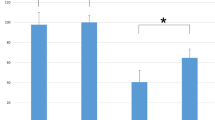
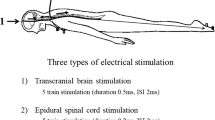
Tetanic stimulation of the peripheral nerve, immediately prior to conducting transcranial electrical stimulation motor evoked potential (TES-MEP), increases MEP amplitudes in both innervated and uninnervated muscles by the stimulated peripheral nerve; this is known as the remote augmentation of MEPs. Nevertheless, the mechanisms underlying the remote augmentation of MEPs remain unclear. Although one hypothesis was that remote augmentation of MEPs results from increased motoneuronal excitability at the spinal cord level, the effect of spinal anterior horn cells has not yet been investigated. We aimed to investigate the effect of tetanic stimulation of the peripheral nerve on spinal cord anterior horn cells by analyzing the F-wave. We included 34 patients who underwent elective spinal surgeries and compared the changes in F-waves and TES-MEPs pre- and post-tetanic stimulation of the median nerve. F-wave analyses were recorded by stimulating the median and tibial nerves. TES-MEPs and F-wave analyses were compared between baseline and post-tetanic stimulation time periods using Wilcoxon signed-rank tests. A significant augmentation of MEPs, independent of the level corresponding to the median nerve, was demonstrated. Furthermore, F-wave persistence was significantly increased not only in the median nerve but also in the tibial nerve after tetanic stimulation of the median nerve. The increased F-wave persistence indicates an increase of re-excited motor units in spinal anterior horn cells. These results confirm the hypothesis that tetanic stimulation of the peripheral nerve may cause remote augmentation of MEPs, primarily by increasing the excitability of the anterior horn cells.






Similar content being viewed by others
Data availability
The datasets during and/or analysed during the current study available from the first author, Yusuke Yamamoto on reasonable request.
Code availability
Not applicable.
References
Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg Spine. 2001;95:161–8. https://doi.org/10.3171/spi.2001.95.2.0161.
Kawaguchi M, Furuya H. Intraoperative spinal cord monitoring of motor function with myogenic motor evoked potentials: a consideration in anesthesia. J Anesth. 2004;18:18–28. https://doi.org/10.1007/s00540-003-0201-9.
Jameson LC, Sloan TB. Neurophysiologic monitoring in neurosurgery. Anesthesiol Clin. 2012;30:311–31. https://doi.org/10.1016/j.anclin.2012.05.005.
Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ. Variability of motor-evoked potentials recorded during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg. 1996;82:744–9. https://doi.org/10.1097/00000539-199604000-00012.
Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430–43. https://doi.org/10.1097/00004691-200210000-00006.
Lotto ML, Banoub M, Schubert A. Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J Neurosurg Anesth. 2004;16:32–42. https://doi.org/10.1097/00008506-200401000-00008.
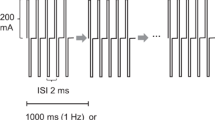
Kakimoto M, Kawaguchi M, Yamamoto Y, Inoue S, Horiuchi T, Nakase H, Sakaki T, Furuya H. Tetanic stimulation of the peripheral nerve before transcranial electrical stimulation can enlarge amplitudes of myogenic motor evoked potentials during general anesthesia with neuromuscular blockade. Anesthesiology. 2005;102:733–8.
Yamamoto Y, Kawaguchi M, Hayashi H, Abe R, Inoue S, Nakase H, Sakaki T, Furuya H. Evaluation of posttetanic motor evoked potentials—the influences of repetitive use, the residual effects of tetanic stimulation to peripheral nerve, and the variability. J Νeurosurg Αnesth. 2010;22:6–10. https://doi.org/10.1097/ANA.0b013e3181b9dd3a.
Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H. The application of tetanic stimulation of the unilateral tibial nerve before transcranial stimulation can augment the amplitudes of myogenic motor-evoked potentials from the muscles in the bilateral upper and lower limbs. Anesth Αnalg. 2008;107:215–20. https://doi.org/10.1213/ane.0b013e318177082e.
Shigematsu H, Kawaguchi M, Hayashi H, Takatani T, Iwata E, Tanaka M, Okuda A, Morimoto Y, Masuda K, Yamamoto Y, Tanaka Y. Post-tetanic transcranial motor evoked potentials augment the amplitude of compound muscle action potentials recorded from innervated and non-innervated muscles. Spine J. 2018;18:740–6. https://doi.org/10.1016/j.spinee.2017.08.249.
Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H. Evaluation of reliability of post-tetanic motor-evoked potential monitoring during spinal surgery under general anesthesia. Spine. 2008;33:E994-e1000. https://doi.org/10.1097/BRS.0b013e318188adfc.
Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–33. https://doi.org/10.1113/jphysiol.2001.012801.
Sun S, Tian FB, Huang SQ, Zhang J, Liang WM. Different effects of tetanic stimulation of facial nerve and ulnar nerve on transcranial electrical stimulation motor-evoked potentials. Int J Clin Exp Med. 2014;7:622–30.
Mercuri B, Wassermann EM, Manganotti P, Ikoma K, Samii A, Hallett M. Cortical modulation of spinal excitability: an F-wave study. Electroen Clin Neuro. 1996;101:16–24. https://doi.org/10.1016/0013-4694(95)00164-6.
Shigematsu H, Kawaguchi M, Hayashi H, Takatani T, Iwata E, Tanaka M, Okuda A, Morimoto Y, Masuda K, Tanaka Y, Tanaka Y. Higher success rate with transcranial electrical stimulation of motor-evoked potentials using constant-voltage stimulation compared with constant-current stimulation in patients undergoing spinal surgery. Spine J. 2017;17:1472–9. https://doi.org/10.1016/j.spinee.2017.05.004.
Tanaka M, Shigematsu H, Kawaguchi M, Hayashi H, Takatani T, Iwata E, Okuda A, Morimoto Y, Kawasaki S, Masuda K, Yamamoto Y, Tanaka Y. Muscle-evoked potentials after electrical stimulation to the brain in patients undergoing spinal surgery are less affected by anesthetic fade with constant-voltage stimulation than with constant-current stimulation. Spine. 2019;44:1492–8. https://doi.org/10.1097/brs.0000000000003166.
Macdonald DB. Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput. 2006;20:347–77. https://doi.org/10.1007/s10877-006-9033-0.
Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroen Clin Neuro. 1996;100:375–83. https://doi.org/10.1016/0168-5597(96)95728-7.
Pechstein U, Cedzich C, Nadstawek J, Schramm J. Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery. 1996;39:335–43. https://doi.org/10.1097/00006123-199608000-00020.
Lyon R, Feiner J, Lieberman JA. Progressive suppression of motor evoked potentials during general anesthesia: the phenomenon of “anesthetic fade.” J Neurosurg Anesth. 2005;17:13–9.
El-Hawary R, Sucato DJ, Sparagana S, McClung A, Van Allen E, Rampy P. Spinal cord monitoring in patients with spinal deformity and neural axis abnormalities: a comparison with adolescent idiopathic scoliosis patients. Spine. 2006;31:E698-706. https://doi.org/10.1097/01.brs.0000232707.98076.37.
Calancie B, Molano MR. Alarm criteria for motor-evoked potentials: what’s wrong with the “presence-or-absence” approach? Spine. 2008;33:406–14. https://doi.org/10.1097/BRS.0b013e3181642a2f.
Journee HL, Polak HE, de Kleuver M, Langeloo DD, Postma AA. Improved neuromonitoring during spinal surgery using double-train transcranial electrical stimulation. Med Biol Eng Comput. 2004;42:110–3. https://doi.org/10.1007/bf02351019.
Tsutsui S, Iwasaki H, Yamada H, Hashizume H, Minamide A, Nakagawa Y, Nishi H, Yoshida M. Augmentation of motor evoked potentials using multi-train transcranial electrical stimulation in intraoperative neurophysiologic monitoring during spinal surgery. J Clin Monit Comput. 2015;29:35–9. https://doi.org/10.1007/s10877-014-9565-7.
MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19:416–29. https://doi.org/10.1097/00004691-200210000-00005.
Kim JS, Choi Y, Jin SH, Kim CH, Park CK, Kim SM, Lee KW, Chung CK, Paek SH. Effect of peripheral nerve tetanic stimulation on the inter-trial variability and accuracy of transcranial motor-evoked potential in brain surgery. Clin Neurophysiol. 2016;127:2208–13. https://doi.org/10.1016/j.clinph.2016.01.018.
Yamamoto Y, Kawaguchi M, Hayashi H, Horiuchi T, Inoue S, Nakase H, Sakaki T, Furuya H. The effects of the neuromuscular blockade levels on amplitudes of posttetanic motor-evoked potentials and movement in response to transcranial stimulation in patients receiving propofol and fentanyl anesthesia. Anesth Analg. 2008;106:930–4. https://doi.org/10.1213/ane.0b013e3181617508.
Ali HH, Savarese JJ. Monitoring of neuromuscular function. Anesthesiology. 1976;45:216–49. https://doi.org/10.1097/00000542-197608000-00009.
Wali FA, Bradshaw EG, Suer AH. Clinical assessment of neuromuscular blockade produced by vecuronium using twitch, train of four, tetanus and post-tetanic twitch responses of the adductor pollicis muscle. Acta Anaesth Belg. 1988;39:35–42.
Andersson G, Ohlin A. Spatial facilitation of motor evoked responses in monitoring during spinal surgery. Clin Neurophysiol. 1999;110:720–4. https://doi.org/10.1016/s1388-2457(98)00049-2.
Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–8. https://doi.org/10.1038/264.
Mesrati F, Vecchierini MF. F-waves: neurophysiology and clinical value. Clin Neurophysiol. 2004;34:217–43. https://doi.org/10.1016/j.neucli.2004.09.005.
Fisher MA. F-waves-physiology and clinical uses. Sci World J. 2007;7:144–60. https://doi.org/10.1100/tsw.2007.49.
Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cerebral Cortex. 1999;9:161–7. https://doi.org/10.1093/cercor/9.2.161.
Jerath NU, Aul E, Reddy CG, Azadeh H, Swenson A, Kimura J. Prolongation of F-wave minimal latency: a sensitive predictor of polyneuropathy. Int J Neurosci. 2015;126:520–5. https://doi.org/10.3109/00207454.2015.1040492.
Nakazumi Y, Watanabe Y. F-wave elicited during voluntary contraction as a monitor of upper motor neuron disorder. Electromyogr Clin Neurophysiol. 1992;32:631–5.
Hagbarth KE. Post-tetanic potentiation of myotatic reflexes in man. J Neurol Neurosurg Psychiatry. 1962;25:1–10. https://doi.org/10.1136/jnnp.25.1.1.
Hultborn H, Nielsen JB. H-reflexes and F-responses are not equally sensitive to changes in motoneuronal excitability. Muscle Nerve. 1995;18:1471–4. https://doi.org/10.1002/mus.880181219.
Guerit JM. Neuromonitoring in the operating room: why, when, and how to monitor? Electroen Clin Neurophysiol. 1998;106:1–21. https://doi.org/10.1016/s0013-4694(97)00077-1.
Rampil IJ, King BS. Volatile anesthetics depress spinal motor neurons. Anesthesiology. 1996;85:129–34. https://doi.org/10.1097/00000542-199607000-00018.
Kammer T, Rehberg B, Menne D, Wartenberg HC, Wenningmann I, Urban BW. Propofol and sevoflurane in subanesthetic concentrations act preferentially on the spinal cord: evidence from multimodal electrophysiological assessment. Anesthesiology. 2002;97:1416–25. https://doi.org/10.1097/00000542-200212000-00013.
Mason P, Owens CA, Hammond DL. Antagonism of the antinocifensive action of halothane by intrathecal administration of GABAA receptor antagonists. Anesthesiology. 1996;84:1205–14. https://doi.org/10.1097/00000542-199605000-00023.
Antognini JF, Carstens E, Buzin V. Isoflurane depresses motoneuron excitability by a direct spinal action: an F-wave study. Anesth Analg. 1999;88:681–5. https://doi.org/10.1097/00000539-199903000-00040.
Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol. 2010;8:2–9. https://doi.org/10.2174/157015910790909502.
Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. https://doi.org/10.1056/NEJMra021261.
Pilurzi G, Ginatempo F, Mercante B, Cattaneo L, Pavesi G, Rothwell JC, Deriu F (2020) Role of cutaneous and proprioceptive inputs in sensorimotor integration and plasticity occurring in the facial primary motor cortex. J Physiol 598: 839–851. https://doi.org/10.1113/JP278877
Fukumoto Y, Bunno Y, Suzuki T (2016) Effect of motor imagery on excitability of spinal neural function and its impact on the accuracy of movement-considering the point at which subjects subjectively determine the 50%MVC point. J Phys Ther Sci 28:3416–3420. https://doi.org/10.1589/jpts.28.3416
Acknowledgements
The authors thank Sayomi Yamamoto, Junko Kato, Shinobu Tado, Tomoshige Miyabayashi, and Ryohei Mizobata for their technical assistance with the TES-MEPs and F-wave trial.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YY: designed the study, collected the data, performed the statistical analysis, and wrote the manuscript. HS: designed the study, collected the data, performed the statistical analysis, and wrote and edited the manuscript. MK: collected patients’ data and reviewed and approved the manuscript. HH: Collected patients’ data and reviewed and approved the manuscript. TT: collected patients’ data and reviewed and approved the manuscript. MT: collected patients’ data and reviewed and approved the manuscript. AO: collected patients’ data and reviewed and approved the manuscript. SK: collected patients’ data and reviewed and approved the manuscript. KM: collected patients’ data and reviewed and approved the manuscript. YS: collected patients’ data and reviewed and approved the manuscript. YT: collected patients’ data, provided critical feedback on the study, and reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. All authors have approved the final article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional review board of Nara Medical University; Reference Number: 1915.
Consent to participate
The requirement for written consent from the study subjects was waived as an “opt-out” process.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamoto, Y., Shigematsu, H., Kawaguchi, M. et al. Tetanic stimulation of the peripheral nerve augments motor evoked potentials by re-exciting spinal anterior horn cells. J Clin Monit Comput 36, 259–270 (2022). https://doi.org/10.1007/s10877-020-00647-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-020-00647-z