Abstract
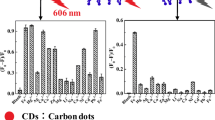
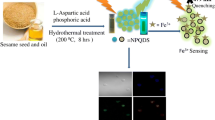
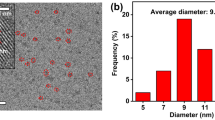
A novel fluorescent probe based on poly(N-isopropyl acrylamide) (p(NIPAAm-co-AAm)) immobilized carbon dots@SiO2 nanoparticles complex was synthesized for ferric iron detection through the chemical crosslinking methods, in which carbon dots@SiO2 nanoparticles complex was characterized by field emission scanning electron microscope, Fourier transform infrared spectroscopy, Ultraviolet–visible spectrophotometer and fluorescence spectrophotometer, respectively. The fluorescence quenching between p(NIPAAm-co-AAm)-CQDs and ferric iron were able to be controlled at different temperatures based on the swelling and shrinking of p(NIPAAm-co-AAm) through changing the temperature, which the principle of the probe was analyzed. The fluorescent probe could detect the ferric iron with a good linear relationship in the range of concentration from 0 to 50 μM and the limit of detection was 0.48 μM at 25 °C.






Similar content being viewed by others
Data availability
The data of this study are available from the corresponding author.
References
Zheng M, Tan HQ, Xie ZG, Zhang LG, Jing XB, Sun ZC (2013) Fast response and high sensitivity europium metal organic framework fluorescent probe with chelating Terpyridine sites for Fe3+ (in English). Acs Appl Mater Inter 5:1078–1083. https://doi.org/10.1021/am302862k
Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders (in English). Nat Rev Neurosci 5:863–873. https://doi.org/10.1038/nrn1537
Tu J, Yang X, Liu H, Chen P, Liu K, Gao J (2022) A ‘on-off-on’ fluorescent probe for sensitive detection of Fe3+ and ascorbic acid by cross-linking agent protected carbon dots. Int J Environ an Ch 102:243–253. https://doi.org/10.1080/03067319.2020.1720010
Bradbury MW (1997) Transport of iron in the blood-brain-cerebrospinal fluid system (in English). J Neurochem 69:443–454. https://doi.org/10.1046/j.1471-4159.1997.69020443.x
Fiedler A, Reinert T, Morawski M, Bruckner G, Arendt T, Butz T (2007) Intracellular iron concentration of neurons with and without perineuronal nets (in English). Nucl Instrum Meth B 260:153–158. https://doi.org/10.1016/j.nimb.2007.02.069
Brugnara C (2003) Iron deficiency and erythropoiesis: New diagnostic approaches (in English). Clin Chem 49:1573–1578. https://doi.org/10.1373/49.10.1573
Neilands JB (1984) Methodology of siderophores, in Siderophores from Microorganisms and Plants. In: Chimiak A, Hider RC, Liu A, Neilands JB, Nomoto K, Sugiura Y (eds) Structure and Bonding. Springer, Berlin Heidelberg, Berlin, pp 1–24. https://doi.org/10.1007/BFb0111309
Hosnedlova B, Sochor J, Baron M, Bjorklund G, Kizek R (2020) Application of nanotechnology based-biosensors in analysis of wine compounds and control of wine quality and safety: A critical review. Crit Rev Food Sci Nutr 60:3271–3289. https://doi.org/10.1080/10408398.2019.1682965
Haas JD, Brownlie Iv T (2001) Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 131:676S-690S. https://doi.org/10.1093/jn/131.2.676S
Lim SY, Shen W, Gao ZQ (2015) Carbon quantum dots and their applications (in English). Chem Soc Rev 44:362–381. https://doi.org/10.1039/c4cs00269e
Li JY et al (2017) One-pot hydrothermal synthesis of carbon dots with efficient up- and down-converted photoluminescence for the sensitive detection of morin in a dual-readout assay (in English). Langmuir 33:1043–1050. https://doi.org/10.1021/acs.langmuir.6b04225
Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective (in English). Nano Res 8:355–381. https://doi.org/10.1007/s12274-014-0644-3
Wang H, Mukherjee S, Ji JH, Banerjee P, Chen QW, Zhou SQ (2017) Biocompatible chitosan-carbon dot hybrid nanogels for NIR-imaging-guided synergistic photothermal-chemo therapy (in English). Acs Appl Mater Inter 9:18639–18649. https://doi.org/10.1021/acsami.7b06062
Lan MH et al (2015) A carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism (in English). Chem Commun 51:15574–15577. https://doi.org/10.1039/c5cc05835j
Li CL et al (2015) Synthesis of photoluminescent carbon dots for the detection of cobalt ions (in English). Rsc Adv 5:2285–2291. https://doi.org/10.1039/c4ra11704b
Ansi VA, Renuka NK (2018) Table sugar derived Carbon dot - a naked eye sensor for toxic Pb2+ ions (in English). Sensor Actuat B-Chem 264:67–75. https://doi.org/10.1016/j.snb.2018.02.167
Luo PJG et al (2013) Carbon “quantum” dots for optical bioimaging (in English). J Mater Chem B 1:2116–2127. https://doi.org/10.1039/c3tb00018d
Roy P et al (2015) Photoluminescent graphene quantum dots for in vivo imaging of apoptotic cells (in English). Nanoscale 7:2504–2510. https://doi.org/10.1039/c4nr07005d
Dhanush C, Aravind MK, Ashokkumar B, Sethuraman MG (2022) Synthesis of blue emissive fluorescent nitrogen doped carbon dots from Annona squamosa fruit extract and their diverse applications in the field of catalysis and bio-imaging. J Photochem Photobiol A Chem 432:114097. https://doi.org/10.1016/j.jphotochem.2022.114097
Kasinathan K, Samayanan S, Marimuthu K, Yim JH (2022) Green synthesis of multicolour fluorescence carbon quantum dots from sugarcane waste: Investigation of mercury (II) ion sensing, and bio-imaging applications. Appl Surf Sci 601:154266. https://doi.org/10.1016/j.apsusc.2022.154266
Liang S, Wang M, Gao W, Luo S, Huang N, Qin Y (2021) Fluorescent carbon dots derived from magnolia withered leaves for promoting growth and fluorescent labeling of bean sprouts. Carbon Trends 4:100063. https://doi.org/10.1016/j.cartre.2021.100063
Ahlawat A, Rana PS, Solanki PR (2021) Studies of photocatalytic and optoelectronic properties of microwave synthesized and polyethyleneimine stabilized carbon quantum dots. Mater Lett 305:130830. https://doi.org/10.1016/j.matlet.2021.130830
Rajapandi S, Pandeeswaran M, Kousalya GN (2022) Novel green synthesis of N-doped carbon dots from fruits of Opuntia ficus Indica as an effective catalyst for the photocatalytic degradation of methyl orange dye and antibacterial studies. Inorg Chem Commun 146:110041. https://doi.org/10.1016/j.inoche.2022.110041
Lu J et al (2022) Photothermal effect of carbon dots for boosted photothermal-assisted photocatalytic water/seawater splitting into hydrogen. Chem Eng J 453:139834. https://doi.org/10.1016/j.cej.2022.139834
Thara CR, Korah BK, Mathew S, John BK, Mathew B (2022) Dual mode detection and sunlight-driven photocatalytic degradation of tetracycline with tailor-made N-doped carbon dots. Environ Res 216:114450. https://doi.org/10.1016/j.envres.2022.114450
Qiu Y, Park K (2012) Environment-sensitive hydrogels for drug delivery (in English). Adv Drug Deliver Rev 64:49–60. https://doi.org/10.1016/j.addr.2012.09.024
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges (in English). Polymer 49:1993–2007. https://doi.org/10.1016/j.polymer.2008.01.027
Basak S, Nandi N, Paul S, Hamley IW, Banerjee A (2017) A tripeptide-based self-shrinking hydrogel for waste-water treatment: removal of toxic organic dyes and lead (Pb2+) ions (in English). Chem Commun 53:5910–5913. https://doi.org/10.1039/c7cc01774j
Koo HJ, So JH, Dickey MD, Velev OD (2011) Towards all-soft matter circuits: prototypes of quasi-liquid devices with memristor characteristics (in English). Adv Mater 23:3559. https://doi.org/10.1002/adma.201101257
Wang B, Xu XD, Wang ZC, Cheng SX, Zhang XZ, Zhu RX (2008) Synthesis and properties of pH and temperature sensitive P(NIPAAm-co-DMAEMA) hydrogels (in English). Colloid Surface B 64:34–41. https://doi.org/10.1016/j.colsurfb.2008.01.001
Satarkar NS, Hilt JZ (2008) Hydrogel nanocomposites as remote-controlled biomaterials (in English). Acta Biomater 4:11–16. https://doi.org/10.1016/j.actbio.2007.07.009
Zhang JT, Keller TF, Bhat R, Garipcan B, Jandt KD (2010) A novel two-level microstructured poly(N-isopropylacrylamide) hydrogel for controlled release (in English). Acta Biomater 6:3890–3898. https://doi.org/10.1016/j.actbio.2010.05.009
Stöber W, Fink A, Bohn E (1986) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interf Sci 26:62–69. https://doi.org/10.1016/0021-9797(68)90272-5
Lin HT, Huang J, Ding LY (2019) Preparation of carbon dots with high-fluorescence quantum yield and their application in dopamine fluorescence probe and cellular imaging. J Nanomater 2019:5037243. https://doi.org/10.1155/2019/5037243
Luo ZT, Lu Y, Somers LA, Johnson ATC (2009) High preparation of macroscopic graphene oxide membranes (in English). J Am Chem Soc 131:898. https://doi.org/10.1021/ja807934n
Wang Y, Kalytchuk S, Zhang Y, Shi HC, Kershaw SV, Rogach AL (2014) Thickness-dependent full-color emission Tunability in a flexible carbon dot Ionogel (in English). J Phys Chem Lett 5:1412–1420. https://doi.org/10.1021/jz5005335
Feng BX, Xu Z, Wang JY, Gai LG (2019) Water-soluble organic polymer/silica composite nanofilms with improved fluorescence quantum yield (in English). J Lumin 211:347–354. https://doi.org/10.1016/j.jlumin.2019.03.066
Heskins M, Guillet JE (1968) Solution properties of Poly(N-isopropylacrylamide). J Macromol Sci Part A Chem 2:1441–1455. https://doi.org/10.1080/10601326808051910
Wang R, Yu F, Liu P, Chen L (2012) A turn-on fluorescent probe based on hydroxylamine oxidation for detecting ferric ion selectively in living cells. Chem Commun 48:5310–5312. https://doi.org/10.1039/C2CC31426F
Xie PH, Guo FQ, Xia RR et al (2014) A rhodamine-dansyl conjugate as a FRET based sensor for Fe3+ in the red spectral region. J Lumin 145:849–854. https://doi.org/10.1016/j.jlumin.2013.09.003
Lee MH, Van Giap T, Kim SH et al (2010) A novel strategy to selectively detect Fe(III) in aqueous media driven by hydrolysis of a rhodamine 6G Schiff base. Chem Commun 46(9):1407–1409. https://doi.org/10.1039/b921526c
Green SA, Morel FMM, Blough NV (1992) Investigation of the electrostatic properties of humic substances by fluorescence quenching. Environ Sci Technol 26:294–302. https://doi.org/10.1021/es00026a008
Acknowledgements
The authors would like to thank Shiyanjia Lab (www.shiyanjia.com) for the FTIR, SEM and AFM measurements.
Funding
This work is supported by Financial Support from Key Technology R&D Program of Shandong Province (2022CXPT052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Ethical approval
Ethics approval is not required for this research.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, H., Jiang, Z., Wang, J. et al. Fluorescent probe based on hydrogel-immobilized carbon dots complex with temperature-trigger for ferric iron detection. J Mater Sci 58, 7602–7612 (2023). https://doi.org/10.1007/s10853-023-08510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08510-7